The Free Internet Journal for Organic Chemistry
|
|
|
- Μελπομένη Βυζάντιος
- 6 χρόνια πριν
- Προβολές:
Transcript
1 The Free Internet Journal for Organic Chemistry Paper Archive for Organic Chemistry Arkivoc 2018, part iii, S1-S6 Synthesis of dihydropyranones and dihydropyrano[2,3- d][1,3]dioxine-diones by cyclization reaction of Meldrum s acid with arylaldehydes and 1,3-dicarbonyls under thermal and ultrasound irradiation Hossein Mehrabi *, Faezeh Najafian-Ashrafi, and Reza Ranjbar-Karimi Department of Chemistry, Vali-e-Asr University of Rafsanjan, 77176, Rafsanjan, Iran mehraby_h@yahoo.com Experimental General. All chemicals were purchased from Aldrich and Merck with high-grade quality, and used without any purification. All melting points were obtained by Bamslead Electrothermal 9200 apparatus and are uncorrected. The reactions were monitored by TLC and all yields refer to isolated products. 1 H and 13 C NMR spectra were recorded in CDCl3 on a Bruker 300 MHz spectrometer. Infrared spectra were recorded on a Bruker FT-IR Equinax-55 spectrophotometer in KBr with absorption in Cm -1. Elemental analyses were performed using a Carlo Erba EA 1108 instrument. All products were characterized by their spectra and physical data. General procedure for the synthesis of dihydropyranone and dihydropyrano[2,3- d][1,3]dioxine-dione derivatives Thermal conditions: Page S1
2 A mixture of the appropriate Meldrum s acid 1 (1.0 mmol), arylaldehydes 2 (1.0 mmol), and various 1,3-dicarbonyls (1.0 mmol) were stirred in H2O:EtOH (4 ml) in the presence of KOH (15%) as a base at 50 o C for an appropriate time. After completion of the reaction, determined by TLC, the solvent was removed under reduced pressure, and the resulting crude product was recrystallized from ethanol to give the pure compounds as a white solid. Ultrasonic- Irradiation conditions: A mixture of the appropriate Meldrum s acid 1 (1.0 mmol), arylaldehydes 2 (1.0 mmol), and various 1,3-dicarbonyls (1.0 mmol) in 2 ml of H2O:EtOH was irradiated under an ultrasonic processor at 25 ± 1 o C and 100 W. After completion of the reaction, determined by TLC, the solvent was removed under reduced pressure, and the resulting crude product was recrystallized from ethanol to give the pure compounds as a white solid. 5-Acetyl-6-methyl-4-phenyl-3,4-dihydro-2H-pyran-2-one (4a). Mp o C ( o C, Lit. 34 ). IR ʋ/cm -1 (KBr): 1724, 1690, 1442, 1358, 1270, 1226, 1186, H NMR (300 MHz, CDCl3): δ 2.10 (s, 3H, COCH3), 2.34 (s, 3H, CH3), 2.68 (dd, j = 2.1 and 15.7 Hz, 1H, CH2), 3.18 (dd, j = 7.2 and 15.7 Hz, 1H, CH2), 4.31 (d, j = 6.6 Hz, 1H, CH), (m, 5H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 18.64, 29.70, 36.84, 37.33, , , , , , , , ppm. 5-Acetyl-6-methyl-4-(o-tolyl)-3,4-dihydro-2H-pyran-2-one (4b). Mp ( o C, Lit. 34 ). IR ʋ/cm -1 (KBr): 1772, 1693, 1622, 1425, 1380, 1353, 1343, 1247, H NMR (300 MHz, CDCl3): δ 2.10 (s, 3H, COCH3), 2.27 (s, 3H, CH3), 2.34 (s, 3H, CH3), 2.67 (dd, j = 2.2 and 15.7 Hz, 1H, CH2), 3.16 (dd, j = 7.2 and 15.7 Hz, 1H, CH2), 4.27 (d, j = 6.5 Hz, 1H, CH), (m, 4H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 18.58, 21.00, 29.64, 36.83, 37.35, , , , , , , , , , ppm. 5-Acetyl-4-(3-chlorophenyl)-6-methyl-3,4-dihydro-2H-pyran-2-one (4c). Mp o C. IR ʋ/cm -1 (KBr): 1762, 1728, 1594, 1572, 1477, 1436, 1392, 1302, 1284, H NMR (300 Page S2
3 MHz, CDCl3): δ 2.10 (s, 3H, COCH3) 2.34 (s, 3H, CH3), 2.67 (dd, j = 2.2 and 15.8 Hz, 1H, CH2), 3.15 (dd, j = 7.0 and 15.8 Hz, 1H, CH2), 4.29 (d, j = 6.4 Hz, 1H, CH), (m, 4H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ = 18.61, 29.70, 36.86, 37.31, , , , , , , , , , ppm. Anal. Calcd for C14H13ClO3 (264.71): C, 63.53; H, Found: C, 63.79; H, 4.97 %. Ethyl 6-methyl-2-oxo-4-phenyl-3,4-dihydro-2H-pyran-5-carboxylate (6a). Mp o C (Yellow oil, Lit. 33 ). IR ʋ/cm -1 (KBr): 1729, 1610, 1583, 1458, 1514, 1377, 1391, 1289, 1201, H NMR (300 MHz, CDCl3): δ 1.08 (t, j = 6.9 Hz, 3H, CH3CH2), 2.40 (s, 3H, CH3), 2.68 (dd, j = 2.1 and 15.9 Hz, 1H, CH2), 3.20 (dd, j = 7.5 and 15.8 Hz, 1H, CH2), 4.05 (q, j = 6.9 Hz, 2H, OCH2), 4.22 (d, j = 6.9 Hz, 1H, CH), (m, 5H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 13.88, 18.43, 36.22, 37.01, 60.37, , , , , , , , ppm. Ethyl 6-methyl-2-oxo-4-(4-(trifluoromethyl)phenyl)-3,4-dihydro-2H-pyran-5- carboxylate (6b) Mp o C. IR ʋ/cm -1 (KBr): 1733, 1712, 1619, 1465, 1376, 1331, 1246, 1160, H NMR (300 MHz, CDCl3): δ = 1.10 (t, j = 6.9 Hz, 3H, CH3CH2), 2.48 (s, 3H, CH3), 2.70 (dd, j = 2.1 and 15.9 Hz, 1H, CH2), 3.23 (dd, j = 7.5 and 15.8 Hz, 1H, CH2), 4.08 (q, j = 6.9 Hz, 2H, OCH2), 4.25 (d, j = 6.9 Hz, 1H, CH), 7.53 (d, j = 8.1 Hz, 2H, ArH), 7.66 (d, j = 8.1 Hz, 2H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 13.91, 19.03, 36.16, 37.01, 61.35, , , , , , , , , ppm. Anal. Calcd for C16H15F3O4 (328.29): C, 58.54; H, Found: C, 58.39; H, 4.58 %. 7,7-Dimethyl-4-(m-tolyl)-4,6,7,8-tetrahydro-2H-chromene-2,5(3H)-dione (8a). Mp o C. IR ʋ/cm -1 (KBr): 1786, 1655, 1373, 1294, 1158, 1147, H NMR (300 MHz, CDCl3): δ 1.06 (s, 3H, CH3), 1.08 (s, 3H, CH3), 2.24 (d, j = 16.0 Hz, 1H, CHH), 2.27 (s, 3H, CH3), 2.33 (d, j = 16.0 Hz, 1H, CHH), 2.51 (d, j = 17.8 Hz, 1H, CHH), 2.62 (d, j = 17.8 Hz, 1H, CHH), 2.70 (dd, j = 1.3 and 15.9 Hz, 1H, CH2), 3.22 (dd, j = 7.8 and 15.9 Hz, 1H, CH2), Page S3
4 4.14 (d, j = 7.5 Hz, 1H, CH), (m, 4H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 21.03, 27.66, 27.83, 32.22, 33.14, 36.58, 49.96, , , , , , , , , , ppm. Anal. Calcd for C18H20O3 (284.36): C, 76.03; H, Found: C, 75.90; H, 7.08 %. 4-(4-Chlorophenyl)-7,7-dimethyl-4,6,7,8-tetrahydro-2H-chromene-2,5(3H)-dione (8b). Mp o C. IR ʋ/cm -1 (KBr): 1772, 1655, 1490, 1373, 1212, H NMR (300 MHz, CDCl3): δ 1.04 (s, 3H, CH3), 1.07 (s, 3H, CH3), 2.23 (d, j = 16.0 Hz, 1H, CHH), 2.31 (d, j = 16.0 Hz, 1H, CHH), 2.56 (d, j = 17.8 Hz, 1H, CHH), 2.61 (d, j = 17.8 Hz, 1H, CHH), 2.71 (dd, j = 1.3 and 16.0 Hz, 1H, CH2), 3.26 (dd, j = 7.9 and 16.1 Hz, 1H, CH2), 4.18 (d, j = 7.5 Hz, 1H, CH), 7.13 (d, j = 7.2 Hz, 2H, ArH), 7.36 (d, j = 7.2 Hz, 2H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 27.71, 27.79, 32.19, 32.60, 36.21, 49.87, , , , , , , , ppm. Anal. Calcd for C17H17ClO3 (304.77): C, 67.00; H, Found: C, 67.12; H, 5.65 %. 5-(4-Chlorophenyl)-6-(1-hydroxy-2-methylprop-1-en-1-yl)-2,2-dimethyl-5,6-dihydro- 4H,7H-pyrano[2,3-d][1,3]dioxine-4,7-dione (10a). Mp o C. IR ʋ/cm -1 (KBr): 1758, 1731, 1605, 1587, 1491, 1391, 1377, 1303, 1285, H NMR (300 MHz, CDCl3): δ 0.54 (s, 6H, 2CH3), 2.25 (s, 6H, 2CH3), 2.44 (m, 1H, CH), 3.44 (d, j = 15.2 Hz, 1H, CH), 4.01 (d, j = 15.2 Hz, 1H, CH), (m, 4H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 18.56, 20.95, 27.61, 42.32, 48.73, 59.78, , , , , , , , , , ppm. Anal. Calcd for C19H19ClO6 (378.81): C, 60.24; H, Found: C, 60.18; H, 5.03 %. 5-(4-Bromophenyl)-6-(1-hydroxy-2-methylprop-1-en-1-yl)-2,2-dimethyl-5,6-dihydro- 4H,7H-pyrano[2,3-d][1,3]dioxine-4,7-dione (10b). Mp o C. IR ʋ/cm -1 (KBr): 1754, 1725, 1514, 1394, 1382, 1362, 1287, H NMR (300 MHz, CDCl3): δ 0.53 (s, 6H, 2CH3), 2.23 (s, 6H, 2CH3), 2.41 (m, 1H, CH), 3.46 (d, j = 14.7 Hz, 1H, CH), 4.00 (d, j = 14.7 Hz, 1H, Page S4
5 CH), 7.02 (d, j = 7.8 Hz, 2H, ArH), 7.16 (d, j = 7.8 Hz, 2H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 19.04, 20.50, 27.72, 48.42, 59.98, , , , , , , , , ppm. Anal. Calcd for C19H19BrO6 (423.26): C, 53.92; H, Found: C, 53.80; H, 4.51 %. 5-(4-Methoxyphenyl)-2,2-dimethyl-5,6-dihydro-4H,7H-pyrano[2,3-d][1,3]dioxine-4,7- dione (12a). Mp o C. IR ʋ/cm -1 (KBr): 1762, 1728, 1610, 1514, 1458, 1391, 1377, 1315, 1289, 1252, H NMR (300 MHz, CDCl3): δ 0.58 (s, 6H, 2CH3), 2.43 (dd, j = 4.4 and 31.5 Hz, 1H, CH2), 3.41 (dd, j = 14.1 and 31.3 Hz, 1H, CH2), 3.69 (s, 3H, OCH3), 4.12 (dd, j = 4.3 and 13.9 Hz, 1H, CH), 6.91 (d, j = 8.7 Hz, 2H, ArH), 7.05 (d, j = 8.7 Hz, 2H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 27.87, 42.56, 47.99, 55.19, 60.35, , , , , , , , ppm. Anal. Calcd for C16H16O6 (304.30): C, 63.15; H, Found: C, 63.27; H, 5.33 %. 2,2-Dimethyl-5-(4-(trifluoromethyl)phenyl)-5,6-dihydro-4H,7H-pyrano[2,3- d][1,3]dioxine-4,7-dione (12b). Mp o C. IR ʋ/cm -1 (KBr): 1731, 1621, 1428, 1394, 1328, 1280, 1171, H NMR (300 MHz, CDCl3): δ 0.50 (s, 6H, 2CH3), 2.42 (dd, j = 4.2 and 31.0 Hz, 1H, CH2), 3.42 (dd, j = 15.1 and 31.5 Hz, 1H, CH2), 4.00 (dd, j = 4.2 and 13.7 Hz, 1H, CH), 7.39 (d, j = 8.1 Hz, 2H, ArH), 7.78 (d, j = 8.1 Hz, 2H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 27.57, 41.71, 48.33, , , , , , , , , ppm. Anal. Calcd for C16H13F3O5 (342.27): C, 56.15; H, Found: C, 56.21; H, 3.79 %. 5-(3-Fluorophenyl)-2,2-dimethyl-5,6-dihydro-4H,7H-pyrano[2,3-d][1,3]dioxine-4,7- dione (12c). Mp o C. IR ʋ/cm -1 (KBr): 1733, 1570, 1475, 1434, 1395, 1304, 1200, H NMR (300 MHz, CDCl3): δ 0.53 (s, 6H, 2CH3), 2.44 (dd, j = 4.2 and 31.1 Hz, 1H, CH2), 3.43 (dd, j = 15.0 and 31.4 Hz, 1H, CH2), 3.98 (dd, j = 4.4 and 13.7 Hz, 1H, CH), (m, 4H, ArH) ppm. 13 C NMR (75 MHz, CDCl3): δ 27.75, 41.94, 48.22, , , Page S5
6 124.60, , , , , , , ppm. Anal. Calcd for C15H13FO5 (292.26): C, 61.64; H, Found: C, 61.57; H, 4.45 %. Page S6
Supporting Information
 Supporting Information Montmorillonite KSF-Catalyzed One-pot, Three-component, Aza-Diels- Alder Reactions of Methylenecyclopropanes With Arylaldehydes and Aromatic Amines Li-Xiong Shao and Min Shi* General
Supporting Information Montmorillonite KSF-Catalyzed One-pot, Three-component, Aza-Diels- Alder Reactions of Methylenecyclopropanes With Arylaldehydes and Aromatic Amines Li-Xiong Shao and Min Shi* General
Supporting Information
 Supporting Information Nano WO3-supported sulfonic acid: New, efficient and high recyclable heterogeneous nano catalyst Ali Amoozadeh* and Salman Rahmani* Department of Chemistry, Semnan University, Semnan,
Supporting Information Nano WO3-supported sulfonic acid: New, efficient and high recyclable heterogeneous nano catalyst Ali Amoozadeh* and Salman Rahmani* Department of Chemistry, Semnan University, Semnan,
A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
Supporting Information
 Supporting Information for AgOTf-catalyzed one-pot reactions of 2-alkynylbenzaldoximes with α,β-unsaturated carbonyl compounds Qiuping Ding 1, Dan Wang 1, Puying Luo* 2, Meiling Liu 1, Shouzhi Pu* 3 and
Supporting Information for AgOTf-catalyzed one-pot reactions of 2-alkynylbenzaldoximes with α,β-unsaturated carbonyl compounds Qiuping Ding 1, Dan Wang 1, Puying Luo* 2, Meiling Liu 1, Shouzhi Pu* 3 and
Supporting Information
 Supporting Information Lewis acid catalyzed ring-opening reactions of methylenecyclopropanes with diphenylphosphine oxide in the presence of sulfur or selenium Min Shi,* Min Jiang and Le-Ping Liu State
Supporting Information Lewis acid catalyzed ring-opening reactions of methylenecyclopropanes with diphenylphosphine oxide in the presence of sulfur or selenium Min Shi,* Min Jiang and Le-Ping Liu State
Supporting Information
 Supporting Information Ceric Ammonium Nitrate (CAN) catalyzed efficient one-pot three component aza-diels-alder reactions for a facile synthesis of tetrahydropyranoquinoline derivatives Ravinder Goud Puligoundla
Supporting Information Ceric Ammonium Nitrate (CAN) catalyzed efficient one-pot three component aza-diels-alder reactions for a facile synthesis of tetrahydropyranoquinoline derivatives Ravinder Goud Puligoundla
Eco-friendly synthesis of diverse and valuable 2-pyridones by catalyst- and solvent-free thermal multicomponent domino reaction
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 SUPPRTIG IFRMATI Eco-friendly synthesis of diverse and valuable 2-pyridones by catalyst-
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 SUPPRTIG IFRMATI Eco-friendly synthesis of diverse and valuable 2-pyridones by catalyst-
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 1 A Facile Way to Synthesize 2H-Chromenes: Reconsideration of the Reaction Mechanism between Salicylic Aldehyde and
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 1 A Facile Way to Synthesize 2H-Chromenes: Reconsideration of the Reaction Mechanism between Salicylic Aldehyde and
Copper-Catalyzed Oxidative Dehydrogenative N-N Bond. Formation for the Synthesis of N,N -Diarylindazol-3-ones
 Electronic Supplementary Material (ESI) for Organic Chemistry Frontiers. This journal is the Partner Organisations 2016 Supporting information Copper-Catalyzed Oxidative Dehydrogenative - Bond Formation
Electronic Supplementary Material (ESI) for Organic Chemistry Frontiers. This journal is the Partner Organisations 2016 Supporting information Copper-Catalyzed Oxidative Dehydrogenative - Bond Formation
Iodine-catalyzed synthesis of sulfur-bridged enaminones and chromones via double C(sp 2 )-H thiolation
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Iodine-catalyzed synthesis of sulfur-bridged enaminones and chromones via
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Iodine-catalyzed synthesis of sulfur-bridged enaminones and chromones via
Electronic Supplementary Information
 Electronic Supplementary Information Unprecedented Carbon-Carbon Bond Cleavage in Nucleophilic Aziridine Ring Opening Reaction, Efficient Ring Transformation of Aziridines to Imidazolidin-4-ones Jin-Yuan
Electronic Supplementary Information Unprecedented Carbon-Carbon Bond Cleavage in Nucleophilic Aziridine Ring Opening Reaction, Efficient Ring Transformation of Aziridines to Imidazolidin-4-ones Jin-Yuan
Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic acids with the assistance of isocyanide
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic
Supporting Information
 Supporting Information Copper/Silver Cocatalyzed Oxidative Coupling of Vinylarenes with ICH 2 CF 3 or ICH 2 CHF 2 Leading to β-cf 3 /CHF 2 -Substituted Ketones Niannian Yi, Hao Zhang, Chonghui Xu, Wei
Supporting Information Copper/Silver Cocatalyzed Oxidative Coupling of Vinylarenes with ICH 2 CF 3 or ICH 2 CHF 2 Leading to β-cf 3 /CHF 2 -Substituted Ketones Niannian Yi, Hao Zhang, Chonghui Xu, Wei
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Synthesis of 3-omosubstituted Pyrroles via Palladium- Catalyzed Intermolecular Oxidative Cyclization
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Synthesis of 3-omosubstituted Pyrroles via Palladium- Catalyzed Intermolecular Oxidative Cyclization
Facile construction of the functionalized 4H-chromene via tandem. benzylation and cyclization. Jinmin Fan and Zhiyong Wang*
 Facile construction of the functionalized 4H-chromene via tandem benzylation and cyclization Jinmin Fan and Zhiyong Wang* Hefei National Laboratory for Physical Science at Microscale, Joint- Lab of Green
Facile construction of the functionalized 4H-chromene via tandem benzylation and cyclization Jinmin Fan and Zhiyong Wang* Hefei National Laboratory for Physical Science at Microscale, Joint- Lab of Green
Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3
 Supporting Information Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3 Shohei Shimokawa, Yuhsuke Kawagoe, Katsuhiko Moriyama, Hideo Togo* Graduate
Supporting Information Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3 Shohei Shimokawa, Yuhsuke Kawagoe, Katsuhiko Moriyama, Hideo Togo* Graduate
Highly enantioselective cascade synthesis of spiropyrazolones. Supporting Information. NMR spectra and HPLC traces
 Highly enantioselective cascade synthesis of spiropyrazolones Alex Zea a, Andrea-Nekane R. Alba a, Andrea Mazzanti b, Albert Moyano a and Ramon Rios a,c * Supporting Information NMR spectra and HPLC traces
Highly enantioselective cascade synthesis of spiropyrazolones Alex Zea a, Andrea-Nekane R. Alba a, Andrea Mazzanti b, Albert Moyano a and Ramon Rios a,c * Supporting Information NMR spectra and HPLC traces
A straightforward metal-free synthesis of 2-substituted thiazolines in air
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information for A straightforward metal-free synthesis of 2-substituted thiazolines
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information for A straightforward metal-free synthesis of 2-substituted thiazolines
Efficient and Simple Zinc mediated Synthesis of 3 Amidoindoles
 Electronic Supplementary Material (ESI) for rganic and Biomolecular Chemistry SUPPRTIG IFRMATI Efficient and Simple Zinc mediated Synthesis of 3 Amidoindoles Anahit Pews-Davtyan and Matthias Beller* Leibniz-Institut
Electronic Supplementary Material (ESI) for rganic and Biomolecular Chemistry SUPPRTIG IFRMATI Efficient and Simple Zinc mediated Synthesis of 3 Amidoindoles Anahit Pews-Davtyan and Matthias Beller* Leibniz-Institut
Supporting Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Silver or Cerium-Promoted Free Radical Cascade Difunctionalization
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Silver or Cerium-Promoted Free Radical Cascade Difunctionalization
SUPPLEMENTARY MATERIAL
 10.1071/CH16014_AC The Authors 2016 Australian Journal of Chemistry 2016, 69(9), 1049-1053 SUPPLEMENTARY MATERIAL A Green Approach for the Synthesis of Novel 7,11-Dihydro-6H-chromeno[3,4- e]isoxazolo[5,4-b]pyridin-6-one
10.1071/CH16014_AC The Authors 2016 Australian Journal of Chemistry 2016, 69(9), 1049-1053 SUPPLEMENTARY MATERIAL A Green Approach for the Synthesis of Novel 7,11-Dihydro-6H-chromeno[3,4- e]isoxazolo[5,4-b]pyridin-6-one
Vilsmeier Haack reagent-promoted formyloxylation of α-chloro-narylacetamides
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 205 Vilsmeier aack reagent-promoted formyloxylation of α-chloro-arylacetamides by formamide Jiann-Jyh
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 205 Vilsmeier aack reagent-promoted formyloxylation of α-chloro-arylacetamides by formamide Jiann-Jyh
Supporting Information. Consecutive hydrazino-ugi-azide reactions: synthesis of acylhydrazines bearing 1,5- disubstituted tetrazoles
 Supporting Information for Consecutive hydrazino-ugi-azide reactions: synthesis of acylhydrazines bearing 1,5- disubstituted tetrazoles Angélica de Fátima S. Barreto*, Veronica Alves dos Santos, and Carlos
Supporting Information for Consecutive hydrazino-ugi-azide reactions: synthesis of acylhydrazines bearing 1,5- disubstituted tetrazoles Angélica de Fátima S. Barreto*, Veronica Alves dos Santos, and Carlos
Supporting Information for
 Supporting Information for An atom-economic route to densely functionalized thiophenes via base-catalyzed rearrangement of 5-propargyl-2H-thiopyran-4(3H)-ones Chunlin Tang a, Jian Qin b, Xingqi Li *a a
Supporting Information for An atom-economic route to densely functionalized thiophenes via base-catalyzed rearrangement of 5-propargyl-2H-thiopyran-4(3H)-ones Chunlin Tang a, Jian Qin b, Xingqi Li *a a
Free Radical Initiated Coupling Reaction of Alcohols and. Alkynes: not C-O but C-C Bond Formation. Context. General information 2. Typical procedure 2
 Free Radical Initiated Coupling Reaction of Alcohols and Alkynes: not C-O but C-C Bond Formation Zhongquan Liu,* Liang Sun, Jianguo Wang, Jie Han, Yankai Zhao, Bo Zhou Institute of Organic Chemistry, Gannan
Free Radical Initiated Coupling Reaction of Alcohols and Alkynes: not C-O but C-C Bond Formation Zhongquan Liu,* Liang Sun, Jianguo Wang, Jie Han, Yankai Zhao, Bo Zhou Institute of Organic Chemistry, Gannan
Supporting Information
 Supporting Information for Lewis acid-catalyzed redox-neutral amination of 2-(3-pyrroline-1-yl)benzaldehydes via intramolecular [1,5]-hydride shift/isomerization reaction Chun-Huan Jiang, Xiantao Lei,
Supporting Information for Lewis acid-catalyzed redox-neutral amination of 2-(3-pyrroline-1-yl)benzaldehydes via intramolecular [1,5]-hydride shift/isomerization reaction Chun-Huan Jiang, Xiantao Lei,
Supporting Information One-Pot Approach to Chiral Chromenes via Enantioselective Organocatalytic Domino Oxa-Michael-Aldol Reaction
 Supporting Information ne-pot Approach to Chiral Chromenes via Enantioselective rganocatalytic Domino xa-michael-aldol Reaction Hao Li, Jian Wang, Timiyin E-Nunu, Liansuo Zu, Wei Jiang, Shaohua Wei, *
Supporting Information ne-pot Approach to Chiral Chromenes via Enantioselective rganocatalytic Domino xa-michael-aldol Reaction Hao Li, Jian Wang, Timiyin E-Nunu, Liansuo Zu, Wei Jiang, Shaohua Wei, *
Supporting Information
 Supporting Information Regioselective Reversal in the Cyclization of 2-Diazo-3,5-dioxo-6-ynoates (ynones, ynamide): Construction of -Pyrones and 3(2H)-Furanones Starting from Identical Materials Feng Wang,
Supporting Information Regioselective Reversal in the Cyclization of 2-Diazo-3,5-dioxo-6-ynoates (ynones, ynamide): Construction of -Pyrones and 3(2H)-Furanones Starting from Identical Materials Feng Wang,
Supporting Information for
 Supporting Information for A ovel Synthesis of luorinated Pyrazoles via Gold(I)-Catalyzed Tandem Aminofluorination of Alkynes in the Presence of Selectfluor Jianqiang Qian, Yunkui Liu,* Jie Zhu, Bo Jiang,
Supporting Information for A ovel Synthesis of luorinated Pyrazoles via Gold(I)-Catalyzed Tandem Aminofluorination of Alkynes in the Presence of Selectfluor Jianqiang Qian, Yunkui Liu,* Jie Zhu, Bo Jiang,
Lewis Acid Catalyzed Propargylation of Arenes with O-Propargyl Trichloroacetimidate: Synthesis of 1,3-Diarylpropynes
 Supporting Information for Lewis Acid Catalyzed Propargylation of Arenes with O-Propargyl Trichloroacetimidate: Synthesis of 1,3-Diarylpropynes Changkun Li and Jianbo Wang* Beijing National Laboratory
Supporting Information for Lewis Acid Catalyzed Propargylation of Arenes with O-Propargyl Trichloroacetimidate: Synthesis of 1,3-Diarylpropynes Changkun Li and Jianbo Wang* Beijing National Laboratory
SUPPORTING INFORMATION. 1. General... S1. 2. General procedure for the synthesis of compounds 3 and 4 in the absence of AgOAc...
 SUPPORTING INFORMATION Table of contents 1. General.... S1 2. General procedure for the synthesis of compounds 3 and 4 in the absence of AgOAc... S2 3. General procedure for the synthesis of compounds
SUPPORTING INFORMATION Table of contents 1. General.... S1 2. General procedure for the synthesis of compounds 3 and 4 in the absence of AgOAc... S2 3. General procedure for the synthesis of compounds
The N,S-Bidentate Ligand Assisted Pd-Catalyzed C(sp 2 )-H. Carbonylation using Langlois Reagent as CO Source. Supporting Information.
 Electronic upplementary Material (EI) for rganic & Biomolecular Chemistry. This journal is The Royal ociety of Chemistry 2018 The,-Bidentate Ligand Assisted Pd-Catalyzed C(sp 2 )-H Carbonylation using
Electronic upplementary Material (EI) for rganic & Biomolecular Chemistry. This journal is The Royal ociety of Chemistry 2018 The,-Bidentate Ligand Assisted Pd-Catalyzed C(sp 2 )-H Carbonylation using
Site-Selective Suzuki-Miyaura Cross-Coupling Reactions of 2,3,4,5-Tetrabromofuran
 1 Site-Selective Suzuki-Miyaura Cross-Coupling Reactions of 2,3,4,5-Tetrabromofuran Munawar Hussain, a Rasheed Ahmad Khera, a Nguyen Thai Hung, a Peter Langer* a,b a Institut für Chemie, Universität Rostock,
1 Site-Selective Suzuki-Miyaura Cross-Coupling Reactions of 2,3,4,5-Tetrabromofuran Munawar Hussain, a Rasheed Ahmad Khera, a Nguyen Thai Hung, a Peter Langer* a,b a Institut für Chemie, Universität Rostock,
Oxyhalogenation of thiols and disulfides into sulfonyl chlorides/ bromides in water using oxone-kx(x= Cl or Br)
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2014 Oxyhalogenation of thiols and disulfides into sulfonyl chlorides/ bromides in water using
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2014 Oxyhalogenation of thiols and disulfides into sulfonyl chlorides/ bromides in water using
Supporting Information. Asymmetric Binary-acid Catalysis with Chiral. Phosphoric Acid and MgF 2 : Catalytic
 Supporting Information Asymmetric Binary-acid Catalysis with Chiral Phosphoric Acid and MgF 2 : Catalytic Enantioselective Friedel-Crafts Reactions of β,γ- Unsaturated-α-Ketoesters Jian Lv, Xin Li, Long
Supporting Information Asymmetric Binary-acid Catalysis with Chiral Phosphoric Acid and MgF 2 : Catalytic Enantioselective Friedel-Crafts Reactions of β,γ- Unsaturated-α-Ketoesters Jian Lv, Xin Li, Long
Supporting Information. A catalyst-free multicomponent domino sequence for the. diastereoselective synthesis of (E)-3-[2-arylcarbonyl-3-
 Supporting Information for A catalyst-free multicomponent domino sequence for the diastereoselective synthesis of (E)-3-[2-arylcarbonyl-3- (arylamino)allyl]chromen-4-ones Pitchaimani Prasanna 1, Pethaiah
Supporting Information for A catalyst-free multicomponent domino sequence for the diastereoselective synthesis of (E)-3-[2-arylcarbonyl-3- (arylamino)allyl]chromen-4-ones Pitchaimani Prasanna 1, Pethaiah
Regioselectivity in the Stille coupling reactions of 3,5- dibromo-2-pyrone.
 Regioselectivity in the Stille coupling reactions of 3,5- dibromo-2-pyrone. Won-Suk Kim, Hyung-Jin Kim and Cheon-Gyu Cho Department of Chemistry, Hanyang University, Seoul 133-791, Korea Experimental Section
Regioselectivity in the Stille coupling reactions of 3,5- dibromo-2-pyrone. Won-Suk Kim, Hyung-Jin Kim and Cheon-Gyu Cho Department of Chemistry, Hanyang University, Seoul 133-791, Korea Experimental Section
Supporting Information. Experimental section
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Copper-mediated radical cross-coupling reaction of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) with phenols or thiophenols. Support Information
 Copper-mediated radical cross-coupling reaction of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) with phenols or thiophenols Dr. Xiao un Tang and Prof. Qing un Chen* Key Laboratory of Organofluorine Chemistry,
Copper-mediated radical cross-coupling reaction of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) with phenols or thiophenols Dr. Xiao un Tang and Prof. Qing un Chen* Key Laboratory of Organofluorine Chemistry,
Supporting Information
 S1 Supporting Information Synthesis of 2-Arylated Hydroxytyrosol Derivatives via Suzuki-Myaura Cross-Coupling Roberta Bernini, a Sandro Cacchi, b* Giancarlo Fabrizi, b* Eleonora Filisti b a Dipartimento
S1 Supporting Information Synthesis of 2-Arylated Hydroxytyrosol Derivatives via Suzuki-Myaura Cross-Coupling Roberta Bernini, a Sandro Cacchi, b* Giancarlo Fabrizi, b* Eleonora Filisti b a Dipartimento
multicomponent synthesis of 5-amino-4-
 Supporting Informartion for Molecular iodine-catalyzed one-pot multicomponent synthesis of 5-amino-4- (arylselanyl)-1h-pyrazoles Camila S. Pires 1, Daniela H. de Oliveira 1, Maria R. B. Pontel 1, Jean
Supporting Informartion for Molecular iodine-catalyzed one-pot multicomponent synthesis of 5-amino-4- (arylselanyl)-1h-pyrazoles Camila S. Pires 1, Daniela H. de Oliveira 1, Maria R. B. Pontel 1, Jean
ESI for. A simple and efficient protocol for the palladium-catalyzed. ligand-free Suzuki reaction at room temperature in aqueous DMF.
 ESI for A simple and efficient protocol for the palladium-catalyzed ligand-free Suzuki reaction at room temperature in aqueous DMF Chun Liu,* Qijian i, Fanying Bao and Jieshan Qiu State Key Laboratory
ESI for A simple and efficient protocol for the palladium-catalyzed ligand-free Suzuki reaction at room temperature in aqueous DMF Chun Liu,* Qijian i, Fanying Bao and Jieshan Qiu State Key Laboratory
Supporting information
 Electronic upplementary Material (EI) for New Journal of Chemistry. This journal is The Royal ociety of Chemistry and the Centre National de la Recherche cientifique 7 upporting information Lipase catalyzed,-addition
Electronic upplementary Material (EI) for New Journal of Chemistry. This journal is The Royal ociety of Chemistry and the Centre National de la Recherche cientifique 7 upporting information Lipase catalyzed,-addition
Metal-free Oxidative Coupling of Amines with Sodium Sulfinates: A Mild Access to Sulfonamides
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting information for Metal-free Oxidative Coupling of Amines with Sodium Sulfinates:
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting information for Metal-free Oxidative Coupling of Amines with Sodium Sulfinates:
9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer catalysts
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer
Aminofluorination of Fluorinated Alkenes
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Synthesis of ɑ CF 3 and ɑ CF 2 H Amines via Aminofluorination of Fluorinated Alkenes Ling Yang,
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Synthesis of ɑ CF 3 and ɑ CF 2 H Amines via Aminofluorination of Fluorinated Alkenes Ling Yang,
Supporting Information
 Supporting Information Wiley-VCH 2014 69451 Weinheim, Germany Copper-Catalyzed Coupling of Oxime Acetates with Sodium Sulfinates: An Efficient Synthesis of Sulfone Derivatives** Xiaodong Tang, Liangbin
Supporting Information Wiley-VCH 2014 69451 Weinheim, Germany Copper-Catalyzed Coupling of Oxime Acetates with Sodium Sulfinates: An Efficient Synthesis of Sulfone Derivatives** Xiaodong Tang, Liangbin
Supplement: Intramolecular N to N acyl migration in conformationally mobile 1 -acyl-1- systems promoted by debenzylation conditions (HCOONH 4
 Cent. Eur. J. Chem. 9(5) 2011 S164-S175 DI: 10.2478/s11532-011-0082-y Central European Journal of Chemistry Supplement: Intramolecular to acyl migration in conformationally mobile 1 -acyl-1- benzyl-3,4
Cent. Eur. J. Chem. 9(5) 2011 S164-S175 DI: 10.2478/s11532-011-0082-y Central European Journal of Chemistry Supplement: Intramolecular to acyl migration in conformationally mobile 1 -acyl-1- benzyl-3,4
Catalyst-free transformation of levulinic acid into pyrrolidinones with formic acid
 Catalyst-free transformation of levulinic acid into pyrrolidinones with formic acid Yawen Wei, a Chao Wang,* a Xue Jiang, a Dong Xue, a Zhao-Tie Liu, a and Jianliang Xiao* a,b a Key Laboratory of Applied
Catalyst-free transformation of levulinic acid into pyrrolidinones with formic acid Yawen Wei, a Chao Wang,* a Xue Jiang, a Dong Xue, a Zhao-Tie Liu, a and Jianliang Xiao* a,b a Key Laboratory of Applied
Supporting Information
 Supporting Information Selective Synthesis of xygen-containing Heterocycles via Tandem Reactions of 1,2-Allenic Ketones with Ethyl 4-Chloroacetoacetate Qiang Wang, a, b Zhouqing Xu b and Xuesen Fan a *
Supporting Information Selective Synthesis of xygen-containing Heterocycles via Tandem Reactions of 1,2-Allenic Ketones with Ethyl 4-Chloroacetoacetate Qiang Wang, a, b Zhouqing Xu b and Xuesen Fan a *
Direct Palladium-Catalyzed Arylations of Aryl Bromides. with 2/9-Substituted Pyrimido[5,4-b]indolizines
![Direct Palladium-Catalyzed Arylations of Aryl Bromides. with 2/9-Substituted Pyrimido[5,4-b]indolizines Direct Palladium-Catalyzed Arylations of Aryl Bromides. with 2/9-Substituted Pyrimido[5,4-b]indolizines](/thumbs/87/95187440.jpg) Direct Palladium-Catalyzed Arylations of Aryl Bromides with 2/9-Substituted Pyrimido[5,4-b]indolizines Min Jiang, Ting Li, Linghua Meng, Chunhao Yang,* Yuyuan Xie*, and Jian Ding State Key Laboratory of
Direct Palladium-Catalyzed Arylations of Aryl Bromides with 2/9-Substituted Pyrimido[5,4-b]indolizines Min Jiang, Ting Li, Linghua Meng, Chunhao Yang,* Yuyuan Xie*, and Jian Ding State Key Laboratory of
Supporting Information for Iron-catalyzed decarboxylative alkenylation of cycloalkanes with arylvinylic carboxylic acids via a radical process
 Supporting Information for Iron-catalyzed decarboxylative alkenylation of cycloalkanes with arylvinylic carboxylic acids via a radical process Jincan Zhao 1, Hong Fang 1, Jianlin Han* 1,2 and Yi Pan* 1
Supporting Information for Iron-catalyzed decarboxylative alkenylation of cycloalkanes with arylvinylic carboxylic acids via a radical process Jincan Zhao 1, Hong Fang 1, Jianlin Han* 1,2 and Yi Pan* 1
Supporting Information
 Supporting Information Wiley-VC 007 9 Weinheim, Germany ew ear Infrared Dyes and Fluorophores Based on Diketopyrrolopyrroles Dipl.-Chem. Georg M. Fischer, Dipl.-Chem. Andreas P. Ehlers, Prof. Dr. Andreas
Supporting Information Wiley-VC 007 9 Weinheim, Germany ew ear Infrared Dyes and Fluorophores Based on Diketopyrrolopyrroles Dipl.-Chem. Georg M. Fischer, Dipl.-Chem. Andreas P. Ehlers, Prof. Dr. Andreas
First DMAP-mediated direct conversion of Morita Baylis. Hillman alcohols into γ-ketoallylphosphonates: Synthesis of
 Supporting Information File 1 for First DMAP-mediated direct conversion of Morita Baylis Hillman alcohols into γ-ketoallylphosphonates: Synthesis of γ-aminoallylphosphonates Marwa Ayadi 1,2, Haitham Elleuch
Supporting Information File 1 for First DMAP-mediated direct conversion of Morita Baylis Hillman alcohols into γ-ketoallylphosphonates: Synthesis of γ-aminoallylphosphonates Marwa Ayadi 1,2, Haitham Elleuch
and Selective Allylic Reduction of Allylic Alcohols and Their Derivatives with Benzyl Alcohol
 FeCl 3 6H 2 O-Catalyzed Disproportionation of Allylic Alcohols and Selective Allylic Reduction of Allylic Alcohols and Their Derivatives with Benzyl Alcohol Jialiang Wang, Wen Huang, Zhengxing Zhang, Xu
FeCl 3 6H 2 O-Catalyzed Disproportionation of Allylic Alcohols and Selective Allylic Reduction of Allylic Alcohols and Their Derivatives with Benzyl Alcohol Jialiang Wang, Wen Huang, Zhengxing Zhang, Xu
Supporting Information. Synthesis and biological evaluation of 2,3-Bis(het)aryl-4-azaindoles Derivatives as protein kinases inhibitors
 Supporting Information Synthesis and biological evaluation of 2,3-Bis(het)aryl-4-azaindoles Derivatives as protein kinases inhibitors Frédéric Pin, a Frédéric Buron, a Fabienne Saab, a Lionel Colliandre,
Supporting Information Synthesis and biological evaluation of 2,3-Bis(het)aryl-4-azaindoles Derivatives as protein kinases inhibitors Frédéric Pin, a Frédéric Buron, a Fabienne Saab, a Lionel Colliandre,
Electronic Supplementary information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary information Sulfonic acid-functionalized MIL-101(Cr) as a highly efficient
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary information Sulfonic acid-functionalized MIL-101(Cr) as a highly efficient
gem-dichloroalkenes for the Construction of 3-Arylchromones
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Pd(OAc)2/S=PPh3 Accelerated Activation of gem-dichloroalkenes for the Construction of 3-Arylchromones
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Pd(OAc)2/S=PPh3 Accelerated Activation of gem-dichloroalkenes for the Construction of 3-Arylchromones
Divergent synthesis of various iminocyclitols from D-ribose
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 205 Divergent synthesis of various iminocyclitols from D-ribose Ramu Petakamsetty,
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 205 Divergent synthesis of various iminocyclitols from D-ribose Ramu Petakamsetty,
Supporting Information. Experimental section
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Anhydrous solvents were transferred by
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Anhydrous solvents were transferred by
Tributylphosphine-Catalyzed Cycloaddition of Aziridines with Carbon Disulfide and Isothiocyanate
 upporting Information Tributylphosphine-Catalyzed Cycloaddition of Aziridines with Carbon Disulfide and Isothiocyanate Jing-Yu Wu, Zhi-Bin Luo, Li-Xin Dai and Xue-Long Hou* a tate Key Laboratory of Organometallic
upporting Information Tributylphosphine-Catalyzed Cycloaddition of Aziridines with Carbon Disulfide and Isothiocyanate Jing-Yu Wu, Zhi-Bin Luo, Li-Xin Dai and Xue-Long Hou* a tate Key Laboratory of Organometallic
Supporting Information. for
 Supporting Information for A general synthetic route to [Cu(X)(NHC)] (NHC = N- heterocyclic carbene, X =Cl, Br, I) complexes Orlando Santoro, Alba Collado, Alexandra M. Z. Slawin, Steven P. Nolan and Catherine
Supporting Information for A general synthetic route to [Cu(X)(NHC)] (NHC = N- heterocyclic carbene, X =Cl, Br, I) complexes Orlando Santoro, Alba Collado, Alexandra M. Z. Slawin, Steven P. Nolan and Catherine
Supporting Information for: Intramolecular Hydrogen Bonding-Assisted Cyclocondensation of. 1,2,3-Triazole Synthesis
 Supporting Information for: Intramolecular Hydrogen Bonding-Assisted Cyclocondensation of α-diazoketones with Various Amines: A Strategy for Catalytic Wolff 1,2,3-Triazole Synthesis Zikun Wang, a Xihe
Supporting Information for: Intramolecular Hydrogen Bonding-Assisted Cyclocondensation of α-diazoketones with Various Amines: A Strategy for Catalytic Wolff 1,2,3-Triazole Synthesis Zikun Wang, a Xihe
Selective mono reduction of bisphosphine
 Griffith Research Online https://research-repository.griffith.edu.au Selective mono reduction of bisphosphine oxides under mild conditions Author etersson, Maria, Loughlin, Wendy, Jenkins, Ian ublished
Griffith Research Online https://research-repository.griffith.edu.au Selective mono reduction of bisphosphine oxides under mild conditions Author etersson, Maria, Loughlin, Wendy, Jenkins, Ian ublished
First Total Synthesis of Antimitotic Compound, (+)-Phomopsidin
 First Total Synthesis of Antimitotic Compound, (+)-Phomopsidin Takahiro Suzuki, a Kenji Usui, a Yoshiharu Miyake, a Michio Namikoshi, b and Masahisa Nakada a, * a Department of Chemistry, School of Science
First Total Synthesis of Antimitotic Compound, (+)-Phomopsidin Takahiro Suzuki, a Kenji Usui, a Yoshiharu Miyake, a Michio Namikoshi, b and Masahisa Nakada a, * a Department of Chemistry, School of Science
Supporting Information
 Supporting Information Metal-catalyzed Stereoselective and Protecting-group-free Synthesis of 1,2-cis-Glycosides Using 4,6-Dimethoxy-1,3,5-triazin-2-yl Glycosides as Glycosyl Donors Tomonari Tanaka,* 1
Supporting Information Metal-catalyzed Stereoselective and Protecting-group-free Synthesis of 1,2-cis-Glycosides Using 4,6-Dimethoxy-1,3,5-triazin-2-yl Glycosides as Glycosyl Donors Tomonari Tanaka,* 1
Supporting Information. Synthesis and biological evaluation of nojirimycin- and
 Supporting Information for Synthesis and biological evaluation of nojirimycin- and pyrrolidine-based trehalase inhibitors Davide Bini 1, Francesca Cardona 2, Matilde Forcella 1, Camilla Parmeggiani 2,3,
Supporting Information for Synthesis and biological evaluation of nojirimycin- and pyrrolidine-based trehalase inhibitors Davide Bini 1, Francesca Cardona 2, Matilde Forcella 1, Camilla Parmeggiani 2,3,
Supporting Information
 Supporting Information Transition-metal-free Ring Expansion Reactions of Indene-1,3-dione: Synthesis of Functionalized Benzoannulated Seven-Membered Ring Compounds Qiyi Yao, Lingkai Kong, Mengdan Wang,
Supporting Information Transition-metal-free Ring Expansion Reactions of Indene-1,3-dione: Synthesis of Functionalized Benzoannulated Seven-Membered Ring Compounds Qiyi Yao, Lingkai Kong, Mengdan Wang,
Supplementary Information for
 Supplementary Information for Organocatalytic Asymmetric Intramolecular [3+2] Cycloaddition: A Straightforward Approach to Access Multiply Substituted Hexahydrochromeno[4,3-b]pyrrolidine Derivatives in
Supplementary Information for Organocatalytic Asymmetric Intramolecular [3+2] Cycloaddition: A Straightforward Approach to Access Multiply Substituted Hexahydrochromeno[4,3-b]pyrrolidine Derivatives in
Cu(I)-Catalyzed Asymmetric Multicomponent Cascade Inverse. Electron-Demand aza-diels-alder/nucleophilic Addition/Ring-Opening
 Cu(I)-Catalyzed Asymmetric Multicomponent Cascade Inverse Electron-Demand aza-diels-alder/nucleophilic Addition/Ring-Opening Reaction Involving 2-Methoxyfurans as Efficient Dienophiles Rong Huang, Xin
Cu(I)-Catalyzed Asymmetric Multicomponent Cascade Inverse Electron-Demand aza-diels-alder/nucleophilic Addition/Ring-Opening Reaction Involving 2-Methoxyfurans as Efficient Dienophiles Rong Huang, Xin
Supporting Information for Synthesis of Fused N-Heterocycles via Tandem C-H Activation
 This journal is The Royal Society of Chemistry 212 Supporting Information for Synthesis of Fused -Heterocycles via Tandem C-H Activation Ge Meng, Hong-Ying iu, Gui-Rong Qu, John S. Fossey, Jian-Ping Li,*
This journal is The Royal Society of Chemistry 212 Supporting Information for Synthesis of Fused -Heterocycles via Tandem C-H Activation Ge Meng, Hong-Ying iu, Gui-Rong Qu, John S. Fossey, Jian-Ping Li,*
Enantioselective Organocatalytic Michael Addition of Isorhodanines. to α, β-unsaturated Aldehydes
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Supplementary information
 Electronic Supplementary Material (ESI) for MedChemComm. This journal is The Royal Society of Chemistry 2015 Supplementary information Synthesis of carboxyimidamide-substituted benzo[c][1,2,5]oxadiazoles
Electronic Supplementary Material (ESI) for MedChemComm. This journal is The Royal Society of Chemistry 2015 Supplementary information Synthesis of carboxyimidamide-substituted benzo[c][1,2,5]oxadiazoles
Efficient Synthesis of Ureas by Direct Palladium-Catalyzed. Oxidative Carbonylation of Amines
 S1 Supporting Information for Efficient Synthesis of Ureas by Direct Palladium-Catalyzed Oxidative Carbonylation of Amines Bartolo Gabriele,*, Giuseppe Salerno, Raffaella Mancuso, and Mirco Costa Dipartimento
S1 Supporting Information for Efficient Synthesis of Ureas by Direct Palladium-Catalyzed Oxidative Carbonylation of Amines Bartolo Gabriele,*, Giuseppe Salerno, Raffaella Mancuso, and Mirco Costa Dipartimento
Supporting Information
 Supporting Information Gold-catalyzed Cycloisomerization of 1,6-Diyne-4-en-3-ols to form Naphthyl Ketone Derivatives. Jian-Jou Lian and Rai-Shung Liu* Department of Chemistry, National Tsing-Hua University,
Supporting Information Gold-catalyzed Cycloisomerization of 1,6-Diyne-4-en-3-ols to form Naphthyl Ketone Derivatives. Jian-Jou Lian and Rai-Shung Liu* Department of Chemistry, National Tsing-Hua University,
Phosphorus Oxychloride as an Efficient Coupling Reagent for the Synthesis of Ester, Amide and Peptide under Mild Conditions
 Supplementary Information for Phosphorus xychloride as an Efficient Coupling Reagent for the Synthesis of Ester, Amide and Peptide under Mild Conditions u Chen,* a,b Xunfu Xu, a Liu Liu, a Guo Tang,* a
Supplementary Information for Phosphorus xychloride as an Efficient Coupling Reagent for the Synthesis of Ester, Amide and Peptide under Mild Conditions u Chen,* a,b Xunfu Xu, a Liu Liu, a Guo Tang,* a
Ionic liquid effect over the Biginelli reaction under homogeneous and heterogeneous catalysis
 Supporting Information for Ionic liquid effect over the Biginelli reaction under homogeneous and heterogeneous catalysis Haline G. O. Alvim, a,b Tatiani B. de Lima, c Heibbe C. B. de Oliveira, a Fabio
Supporting Information for Ionic liquid effect over the Biginelli reaction under homogeneous and heterogeneous catalysis Haline G. O. Alvim, a,b Tatiani B. de Lima, c Heibbe C. B. de Oliveira, a Fabio
Experimental procedure
 Supporting Information for Direct electrophilic N-trifluoromethylthiolation of amines with trifluoromethanesulfenamide Sébastien Alazet 1,2, Kevin Ollivier 1 and Thierry Billard* 1,2 Address: 1 Institute
Supporting Information for Direct electrophilic N-trifluoromethylthiolation of amines with trifluoromethanesulfenamide Sébastien Alazet 1,2, Kevin Ollivier 1 and Thierry Billard* 1,2 Address: 1 Institute
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry Electronic Supplementary Information (ESI) For Iron-Catalysed xidative Amidation of Alcohols with Amines Silvia Gaspa, a Andrea
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry Electronic Supplementary Information (ESI) For Iron-Catalysed xidative Amidation of Alcohols with Amines Silvia Gaspa, a Andrea
Supporting Information Synthesis of cyclometalated 1,3,5-triphenylpyrazole palladium dimer and its activity towards cross coupling reactions
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 215 Supporting Information Synthesis of cyclometalated 1,3,5-triphenylpyrazole palladium
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 215 Supporting Information Synthesis of cyclometalated 1,3,5-triphenylpyrazole palladium
Supplementary Figure S1. Single X-ray structure 3a at probability ellipsoids of 20%.
 Supplementary Figure S1. Single X-ray structure 3a at probability ellipsoids of 20%. S1 Supplementary Figure S2. Single X-ray structure 5a at probability ellipsoids of 20%. S2 H 15 Ph Ac Ac I AcH Ph Ac
Supplementary Figure S1. Single X-ray structure 3a at probability ellipsoids of 20%. S1 Supplementary Figure S2. Single X-ray structure 5a at probability ellipsoids of 20%. S2 H 15 Ph Ac Ac I AcH Ph Ac
Construction of Cyclic Sulfamidates Bearing Two gem-diaryl Stereocenters through a Rhodium-Catalyzed Stepwise Asymmetric Arylation Protocol
 Supporting Information for: Construction of Cyclic Sulfamidates Bearing Two gem-diaryl Stereocenters through a Rhodium-Catalyzed Stepwise Asymmetric Arylation Protocol Yu-Fang Zhang, Diao Chen, Wen-Wen
Supporting Information for: Construction of Cyclic Sulfamidates Bearing Two gem-diaryl Stereocenters through a Rhodium-Catalyzed Stepwise Asymmetric Arylation Protocol Yu-Fang Zhang, Diao Chen, Wen-Wen
Supporting Information
 Supporting Information An Approach to 3,6-Disubstituted 2,5-Dioxybenzoquinones via Two Sequential Suzuki Couplings. Three-step Synthesis of Leucomelone Xianwen Gan, Wei Jiang, Wei Wang,,,* Lihong Hu,,*
Supporting Information An Approach to 3,6-Disubstituted 2,5-Dioxybenzoquinones via Two Sequential Suzuki Couplings. Three-step Synthesis of Leucomelone Xianwen Gan, Wei Jiang, Wei Wang,,,* Lihong Hu,,*
Room Temperature Highly Diastereoselective Zn-Mediated. Allylation of Chiral N-tert-Butanesulfinyl Imines: Remarkable Reaction Condition Controlled
 Supporting Information for: Room Temperature Highly Diastereoselective Zn-Mediated Allylation of Chiral N-tert-Butanesulfinyl Imines: Remarkable Reaction Condition Controlled Stereoselectivity Reversal
Supporting Information for: Room Temperature Highly Diastereoselective Zn-Mediated Allylation of Chiral N-tert-Butanesulfinyl Imines: Remarkable Reaction Condition Controlled Stereoselectivity Reversal
Rhodium-Catalyzed Oxidative Decarbonylative Heck-type Coupling of Aromatic Aldehydes with Terminal Alkenes
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Rhodium-Catalyzed Oxidative Decarbonylative Heck-type
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Rhodium-Catalyzed Oxidative Decarbonylative Heck-type
Supporting Information for
 Supporting Information for Palladium-Catalyzed C-H Bond Functionalization of C6-Arylpurines Hai-Ming Guo,* Wei-Hao Rao, Hong-Ying iu, Li-Li Jiang, Ge ng, Jia-Jia Jin, Xi-ing Yang, and Gui-Rong Qu* College
Supporting Information for Palladium-Catalyzed C-H Bond Functionalization of C6-Arylpurines Hai-Ming Guo,* Wei-Hao Rao, Hong-Ying iu, Li-Li Jiang, Ge ng, Jia-Jia Jin, Xi-ing Yang, and Gui-Rong Qu* College
Supporting Information
 Supporting Information Visible Light Initiated Hantzsch Synthesis of 2,5-Diaryl Substituted Pyrroles at Ambient Conditions Tao Lei, Wen-Qiang Liu, Jian Li, Mao-Yong Huang, Bing Yang, Qing-Yuan Meng, Bin
Supporting Information Visible Light Initiated Hantzsch Synthesis of 2,5-Diaryl Substituted Pyrroles at Ambient Conditions Tao Lei, Wen-Qiang Liu, Jian Li, Mao-Yong Huang, Bing Yang, Qing-Yuan Meng, Bin
Supporting Information
 S1 Supporting Information Benzannulation from Alkyne without tallic Catalysts at Room Temperature to 100 o C Tienan Jin,* Fan Yang and Yoshinori Yamamoto* Department of Chemistry, Graduate School of Science,
S1 Supporting Information Benzannulation from Alkyne without tallic Catalysts at Room Temperature to 100 o C Tienan Jin,* Fan Yang and Yoshinori Yamamoto* Department of Chemistry, Graduate School of Science,
Peptidomimetics as Protein Arginine Deiminase 4 (PAD4) Inhibitors
 Peptidomimetics as Protein Arginine Deiminase 4 (PAD4) Inhibitors Andrea Trabocchi a, icolino Pala b, Ilga Krimmelbein c, Gloria Menchi a, Antonio Guarna a, Mario Sechi b, Tobias Dreker c, Andrea Scozzafava
Peptidomimetics as Protein Arginine Deiminase 4 (PAD4) Inhibitors Andrea Trabocchi a, icolino Pala b, Ilga Krimmelbein c, Gloria Menchi a, Antonio Guarna a, Mario Sechi b, Tobias Dreker c, Andrea Scozzafava
Fischer Indole Synthesis in Low Melting Mixtures
 Fischer Indole Synthesis in Low Melting Mixtures Sangram Gore, a,b Sundarababu Baskaran* a and Burkhard König* b Supporting Information Table of Contents: General Information General Procedure for the
Fischer Indole Synthesis in Low Melting Mixtures Sangram Gore, a,b Sundarababu Baskaran* a and Burkhard König* b Supporting Information Table of Contents: General Information General Procedure for the
SUPPLEMENTARY DATA. Waste-to-useful: Biowaste-derived heterogeneous catalyst for a green and sustainable Henry reaction
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 209 SUPPLEMENTARY DATA Waste-to-useful:
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 209 SUPPLEMENTARY DATA Waste-to-useful:
Supporting Information
 1 upporting Information Rhodium(III)-Catalyzed rtho Halogenations of N-Acylsulfoximines and ynthetic Applications toward Functionalized ulfoximine Derivatives Ying Cheng, Wanrong Dong, Kanniyappan Parthasarathy,
1 upporting Information Rhodium(III)-Catalyzed rtho Halogenations of N-Acylsulfoximines and ynthetic Applications toward Functionalized ulfoximine Derivatives Ying Cheng, Wanrong Dong, Kanniyappan Parthasarathy,
Available online at
 J. Serb. Chem. Soc. 76 (12) S1 S5 (2011) Supplementary material SUPPLEMENTARY MATERIAL TO Synthesis of quinoline-attached furan-2(3h)-ones having anti-inflammatory and antibacterial properties with reduced
J. Serb. Chem. Soc. 76 (12) S1 S5 (2011) Supplementary material SUPPLEMENTARY MATERIAL TO Synthesis of quinoline-attached furan-2(3h)-ones having anti-inflammatory and antibacterial properties with reduced
Synthesis of novel 1,2,3-triazolyl derivatives of pregnane, androstane and D-homoandrostane. Tandem Click reaction/cu-catalyzed D-homo rearrangement
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 Supporting Information Synthesis of novel 1,2,3-triazolyl derivatives of
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 Supporting Information Synthesis of novel 1,2,3-triazolyl derivatives of
Supplementary Figure 1. (X-ray structures of 6p and 7f) O N. Br 6p
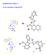 Supplementary Figure 1 (X-ray structures of 6p and 7f) Me Br 6p 6p Supplementary Figures 2-68 (MR Spectra) Supplementary Figure 2. 1 H MR of the 6a Supplementary Figure 3. 13 C MR of the 6a Supplementary
Supplementary Figure 1 (X-ray structures of 6p and 7f) Me Br 6p 6p Supplementary Figures 2-68 (MR Spectra) Supplementary Figure 2. 1 H MR of the 6a Supplementary Figure 3. 13 C MR of the 6a Supplementary
Hiyama Cross-Coupling of Chloro-, Fluoroand Methoxy- pyridyl trimethylsilanes : Room-temperature Novel Access to Functional Bi(het)aryl
 Hiyama Cross-Coupling of Chloro-, Fluoroand Methoxy- pyridyl trimethylsilanes : Room-temperature Novel Access to Functional Bi(het)aryl Philippe Pierrat, Philippe Gros* and Yves Fort Synthèse Organométallique
Hiyama Cross-Coupling of Chloro-, Fluoroand Methoxy- pyridyl trimethylsilanes : Room-temperature Novel Access to Functional Bi(het)aryl Philippe Pierrat, Philippe Gros* and Yves Fort Synthèse Organométallique
Fluorinative Ring-opening of Cyclopropanes by Hypervalent Iodine Reagents. An Efficient Method for 1,3- Oxyfluorination and 1,3-Difluorination
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Supporting Information Fluorinative Ring-opening of Cyclopropanes by Hypervalent Iodine
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Supporting Information Fluorinative Ring-opening of Cyclopropanes by Hypervalent Iodine
Supporting Information for Fe-Catalyzed Reductive Coupling of Unactivated Alkenes with. β-nitroalkenes. Contents. 1. General Information S2
 Supporting Information for Fe-Catalyzed Reductive Coupling of Unactivated Alkenes with β-nitroalkenes Jing Zheng, Dahai Wang, and Sunliang Cui* College of Pharmaceutical Sciences, Zhejiang University,
Supporting Information for Fe-Catalyzed Reductive Coupling of Unactivated Alkenes with β-nitroalkenes Jing Zheng, Dahai Wang, and Sunliang Cui* College of Pharmaceutical Sciences, Zhejiang University,
Eur. J. Org. Chem WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006 ISSN X SUPPORTING INFORMATION
 Eur. J. rg. Chem. 2006 WILEY-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 ISSN 1434 193X SUPPRTING INFRMATIN Title: ne-pot Synthesis of xo Acid Derivatives by Rh I -Catalyzed Chelation-Assisted Hydroacylation
Eur. J. rg. Chem. 2006 WILEY-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 ISSN 1434 193X SUPPRTING INFRMATIN Title: ne-pot Synthesis of xo Acid Derivatives by Rh I -Catalyzed Chelation-Assisted Hydroacylation
Synthesis of Imines from Amines in Aliphatic Alcohols on Pd/ZrO 2 Catalyst at Ambient Conditions
 This journal is The Royal Society of Chemistry 213 Synthesis of Imines from Amines in Aliphatic Alcohols on Pd/ZrO 2 Catalyst at Ambient Conditions Wenjing Cui, a Bao Zhaorigetu,* a Meilin Jia, a and Wulan
This journal is The Royal Society of Chemistry 213 Synthesis of Imines from Amines in Aliphatic Alcohols on Pd/ZrO 2 Catalyst at Ambient Conditions Wenjing Cui, a Bao Zhaorigetu,* a Meilin Jia, a and Wulan
