C C AIBN. Scheme Pd /Ag. α- C H CHINESE JOURNAL OF APPLIED CHEMISTRY Aug b. CuI /DTBP N- O664
|
|
|
- Σίβύλλα Δυοβουνιώτης
- 6 χρόνια πριν
- Προβολές:
Transcript
1 32 8 Vol. 32 Iss CHINESE JOURNAL OF APPLIED CHEMISTRY Aug a a b* a a a a a b CuI /DTBP N- α- α- α- C H O664 A DOI /j. issn C C AIBN 11 N- 4 α- N- Scheme / N- - Pd /Ag / 120 α-c H - α- α Scheme 2 Scheme 1 Several representative pharmaceutical oxindoles JDZ Tel /Fax stang@ jsu. edu. cn
2 8 885 α- Scheme 2 Synthesis of oxindoles bearing α-cyano quarterary carbon center AIBN AVANCE 400 MHz Bruker TMS GC-MS-QP2010 XT5A 1. 2 N a mg 0. 5 mmol N- 1a 2 ml 9. 6 mg mmol 244 mg 1. 0 mmol mg 1. 0 mmol h TLC - 3a 3a mp 158 ~ H NMR 400 MHz CDCl 3 δ ~ m 2H t J = 7. 6 Hz 1H d J = 8. 0 Hz 1H s 3H d J = Hz 1H d J = 14. 4Hz 1H ~ m. 10H s 3H 13 C NMR 101 MHz CDCl 3 δ IR KBr σ /cm HRMS m /z ESI C 18 H 23 N 2 O M + H b mp 145 ~ H NMR 400 MHz CDCl 3 δ s 1H d J = 7. 6 Hz 1H d J = 8. 0 Hz 1H s 3H s 3H d J = Hz 1H 2 11 d J = Hz 1H ~ m 10H s 3H 13 C NMR 101 MHz CDCl 3 δ IR KBr σ /cm HRMS m /z ESI C 19 H 25 N 2 O M + H c mp 150 ~ H NMR 400 MHz CDCl 3 δ ~ m 2H ~ m 1H s 3H d Hz 1H d J = Hz 1H ~ m 10H s 3H 13 C NMR 101 MHz CDCl 3 δ d J = 241 Hz d J = 7. 8 Hz d J = Hz d J = 7. 1 Hz IR KBr σ /cm
3 HRMS m /z ESI C 18 H 22 FN 2 O M + H d mp 140 ~ H NMR 400 MHz CDCl 3 δ dd J = 8. 4 Hz 1. 6 Hz 1H s 1H d J = 8. 4 Hz 1H q J = 6. 8 Hz 2H s 3H d J = Hz 1H d J = Hz 1H ~ m 10H t J = 7. 2 Hz 3H s 3H 13 C NMR 101 MHz CDCl 3 δ IR KBr σ /cm HRMS m /z ESI C 21 H 27 N 2 O 3 M + H f mp 100 ~ H NMR 400 MHz CDCl 3 δ ~ m 2H t J = 6. 0 Hz 1H d J = 6. 4 Hz 1H s 3H d J = Hz 1H d J = H s 3H s 3H s 3H 13 C NMR 101 MHz CDCl 3 δ IR KBr σ /cm HRMS m /z ESI C 15 H 19 N 2 O M + H g mp 80 ~ 81 1 H NMR 400 MHz CDCl 3 δ ~ m 7H t J = 7. 2 Hz 1H d J = 8. 0 Hz 2H d J = Hz 1H d J = Hz 1H d J = Hz 1H d J = Hz 1H s 3H s 3H s 3H 13 C NMR 101 MHz CDCl 3 δ MS IR KBr σ /cm HRMS m /z ESI C 21 H 23 N 2 O M + H h mp 121 ~ H NMR 400 MHz CDCl 3 δ ~ m 7H t J = 8. 0 Hz 1H d J = 8. 0 Hz 1H s 3H d J = Hz 1H d J = Hz 1H s 3H s 3H 13 C NMR 101 MHz CDCl 3 δ IR KBr σ /cm HRMS m /z ESI C 17 H 21 N 2 O M + H i 1 H NMR 400 MHz CDCl 3 δ m 2H t J = 7. 6 Hz 1H d J = 8. 4 Hz 1H d J = Hz 1H d J = Hz 1H s 3H s 2H s 3H s 3H s 3H 13 C NMR 101 MHz CDCl 3 δ IR KBr σ /cm HRMS m /z ESI C 17 H 21 N 2 O 3 M + H + 3j 1 H NMR 400 MHz CDCl 3 δ d J = 7. 2 Hz 1H d J = 7. 6 Hz 1H t J = 7. 6 Hz 1H ~ m 2H ~ m 2H d J = Hz 1H d J = Hz 1H ~ m 2H s 3H s 3H s 3H 13 C NMR 101 MHz CDCl 3 δ IR KBr σ /cm HRMS m /z ESI C 17 H 21 N 2 O M + H
4 k mp 128 ~ H NMR 400 MHz CDCl 3 δ ~ m 2H d J = 8. 0 Hz 1H s 3H d J = Hz 1H d J = Hz 1H s 3H s 3H s 3H 13 C NMR 101 MHz CDCl 3 δ IR KBr σ /cm HRMS m /z ESI C 15 H 18 ClN 2 O M + H l mp 107 ~ H NMR 400 MHz CDCl 3 δ ~ m 2H ~ m 1H s 3H d J = Hz 1H d J = Hz 1H s 3H s 3H s 3H 13 C NMR 101 MHz CDCl 3 δ d J = Hz d J = 23. 4Hz d J = Hz IR KBr σ /cm HRMS m /z ESI C 15 H 18 FN 2 O M + H m mp 114 ~ H NMR 400 MHz CDCl 3 δ d J = 8. 4 Hz 1H s 1H d J = 8. 4 Hz 1H s 3H d J = Hz 1H d J = Hz 1H s 3H s 6H 13 C NMR 101 MHz CDCl 3 δ q J = 3. 9 Hz q J = Hz q J = 3. 5 Hz IR KBr σ /cm HRMS m /z ESI C 16 H 18 F 3 N 2 O M + H c 150 mg 0. 5 mmol LiAlH 4 76 mg 2. 0 mmol 10 ml N 2 1 h 0. 5 h THF /H 2 O ml h 1 mol /L HCl 5 ml 5 min K 2 CO 3 Na 2 SO mg 73% 4 1 H NMR 400 MHz CDCl 3 δ ~ m 2H dd J = 8. 0 Hz 4. 0 Hz 1H s 1H s 3H d J = Hz 1H d J = Hz 1H br 1H ~ m 10H s 3H ~ m 2H 13 C NMR 101 MHz CDCl 3 δ d J = Hz d J = Hz d J = Hz d J = 7. 1 Hz HRMS m /z ESI C 18 H 26 FN 2 M + H N- -N- 1a 1 1-2a 1 23% 3a 1 entry 1 92% 3a 1 entries 2 ~ 5 FeSO 4 7H 2 O Fe NH 4 2 SO 4 2 6H 2 O 65%
5 % 1 entries 6 ~ 8 DTBP TBHP DTBP 76% 1 entry 9 70% 1 entry 10 PhI OAc 2 PhI OTFA 2 DTBP 1 entries 11 ~ 12 14% 1 entry 13 90% 1 entry 14 1 entries 15 ~ % 1 entry % 1 entry 18 N mmol a- 1 mmol CuI 0. 05mmol DTBP 1 mmol h Table 1 1 Screening of optimal reaction conditions a Entry Metal Oxidant Solvent Yield /% b 1 none DTBP CH 3 CN 23 2 CuCl DTBP CH 3 CN 85 3 CuBr DTBP CH 3 CN 74 4 CuI DTBP CH 3 CN 92 5 CuCN DTBP CH 3 CN 87 6 FeSO 4 7H 2 O DTBP CH 3 CN 65 7 FeBr 2 DTBP CH 3 CN 75 8 Fe NH 4 2 SO 4 2 6H 2 O DTBP CH 3 CN 70 9 CuI TBHP CH 3 CN CuI K 2 S 2 O 8 CH 3 CN CuI PhI OAc 2 CH 3 CN CuI PhI OTFA 2 CH 3 CN CuI none CH 3 CN CuI DTBP dioxane CuI DTBP toluene CuI DTBP DCE c CuI DTBP CH 3 CN d CuI DTBP CH 3 CN 64 a. Reaction conditions 1a 0. 5 mmol 2a 1 mmol oxidant 1 mmol metal mmol solvent 2 ml at 80 for 12 h. DTBP = Di-tert-butyl peroxide TBHP = tert-butyl hydrogenoxide 70% aqueous solution DCE = 1 2-dichloroethane b. isolated yield c. 60 d Scheme a N- Me H F CO 2 Et N- 95% 3e
6 8 889 AIBN 2 2'- -2 2'- 81% 3f AIBN N- N 70% 3g CH 2 OAc 73% 66% 3h 3i 3j Scheme 3 Scope of N-arylacrylamide 1 Reaction conditions mmol 2 1 mmol DTBP 1 mmol CuI mmol and CH 3 CN 2 ml at 80 for 12 h. a. run on 5 mmol scale c LiALH 4 73% 4 4 Scheme 4 Scheme 4 Synthetic transformation of oxindole 3c Reagent and conditions a LiAlH 4 4 equiv THF 1 h then reflux 0. 5 h
7 Scheme 5 Proposed mechanism for the formation of oxindoles Scheme 5 1a A A N- B C C Cu Ⅰ t BuO-Cu Ⅱ t BuO 8 3a 3 CuI 5% DTBP 2 80 N- 1 Posner G H. Multicomponent One-pot Annulations Forming 3 to 6 Bonds J. Chem Rev Lu L Q Chen J R Xiao W J. Development of Cascade Reactions for the Concise Construction of Diverse Heterocyclic Architectures J. Acc Chem Res Mai W Wang J Yang L et al. Progress in Synthesis of Oxindoles by Radical Addition-Cyclization J. Chinese J Org Chem Tang S Zhou D Deng Y et al. Copper-catalyzed Meerwein Carboarylation of Alkenes with Anilines to Form 3-Benzyl-3- Alkyloxindole J. Sci China Chem Shen T Yuan Y Jiao N. Metal-Free Nitro-Carbocyclization of Activated Alkenes A Direct Approach to Synthesize Oxindoles by Cascade C N and C C Bond Formation J. Chem Commun Zhou M Song R Ouyang X et al. Metal-free Oxidative Tandem Coupling of Activated Alkenes with Carbonyl C Sp2 -H Bonds and Aryl C Sp2 -H Bonds using TBHP J. Chem Sci Ouyang X Song R Li J. Iron-Catalyzed Oxidative 1 2-Carboacylation of Activated Alkenes with Alcohols A Tandem Route to 3-2-Oxoethyl indolin-2-ones J. Eur J Org Chem Dai Q Yu J Jiang Y et al. The Carbomethylation of Arylacrylamides Leading to 3-Ethyl-3-Substituted Indolin-2-One by Cascade Radical Addition /Cyclization J. Chem Commun Zhou M Wang C Song R et al. Oxidative 1 2-Difunctionalization of Activated Alkenes with Benzylic C sp3 -H Bonds and Aryl C sp2 -H Bonds J. Chem Commun Chen J Yu X Xiao W. Tandem Radical Cyclization of N-Arylacrylamides An Emerging Platform for the Construction of 3 3-Disubstituted Oxindoles J. Synthesis
8 Yu W Sit W N Lai K et al. Palladium-Catalyzed Oxidative Ethoxycarbonylation of Aromatic C H Bond with Diethyl Azodicarboxylate J. J Am Chem Soc Klein J Taylor R. Transition-Metal-Mediated Routes to 3 3-Disubstituted Oxindoles through Anilide Cyclisation J. Eur J Org Chem Wu T Mu X Liu G S. Palladium-Catalyzed Oxidative Arylalkylation of Activated Alkenes Dual C H Bond Cleavage of an Arene and Acetonitrile J. Angew Chem Int Ed Li J Wang Z Wu N et al. Oxindoles J. Chem Commun Radical Cascade Cyanomethylation of Activated Alkenes to Construct Cyano Substituted 15 Pan C Zhang H Zhu C. Fe-promoted Radical Cyanomethylation / Arylation of Arylacrylamides to Access Oxindoles via Cleavage of the sp3c H of Acetonitrile and the sp2c H of the Phenyl Group J. Org Biomol Chem Tang S Yu Q Peng P et al. Palladium-catalyzed Carbonylative Annulation Reaction of 2-1-Alkynyl benzenamines Selective Synthesis of 3- Halomethylene indolin-2-ones J. Org Lett Tang S Zhou D Wang Y. Metal-Free Meerwein Carboarylation of Alkenes with Anilines A Straightforward Approach to 3-Benzyl-3-alkyloxindoles J. Eur J Org Chem Tang S Li S Zhou D et al. Stereoselective C sp 3 C sp 2 Negishi Coupling of 2-Amido-1-phenylpropyl zinc Compounds Through the Steric Control of β-amido Group J. Sci Chinese Chem Copper-catalyzed Cyanoalkylation of Alkenes to Form Cyano- containing Oxindoles LI Zhihao a TANG Shi a b* ZHOU Dong a DENG Youlin a LI Yuhua a LI Shuhua a a Key Laboratory of Hu'nan Forest Product and Chemical Industry Engineering Jishou University Zhangjiajie Hu'nan China b College of Chemistry and Chemical Engineering Central South University Changsha China Abstract A practical mild and highly efficient cyanoalkylation reaction of activated alkenes by copper catalysis has been developed. In the presence of CuI /DTBP Di-tert-butyl peroxide N-arylacrylamide undergoes radical cyclization smoothly and affords a series of pharmaceutically important oxindoles bearing an α-cyano quaternary carbon center. The protocol features broad substrate scope simplicity of operation and handling and inexpensive catalytic systems. In addition the synthetic application and possible mechanism process in the cyclization reaction were also demonstrated. Keywords α-cyano azo compounds cyano-containing oxindoles C H cyclization radical copper catalysis Received Revised Accepted Supported by the National Natural Science Foundation of China NSFC No Key Laboratory of Hunan Forest Product and Chemical Industry Engineering No. JDZ Research-Based Learning and Innovative Experiment Project of Jishou University Corresponding author TANG Shi associate professor Tel /Fax stang@ jsu. edu. cn Research interests transitionmetal catalysis and synthesis
AIBN Scheme 1 85% CHINESE JOURNAL OF APPLIED CHEMISTRY Nov b AIBN N- 53% ~ 85% C H O664
 32 11 Vol. 32 Iss. 11 2015 11 CHINESE JOURNAL OF APPLIED CHEMISTRY Nov. 2015 a b* a a a a a b a 416000 b 410083 AIBN N- 53% ~ 85% C H O664 A DOI 10. 11944 /j. issn. 1000-0518. 2015. 11. 150108 1000-0518
32 11 Vol. 32 Iss. 11 2015 11 CHINESE JOURNAL OF APPLIED CHEMISTRY Nov. 2015 a b* a a a a a b a 416000 b 410083 AIBN N- 53% ~ 85% C H O664 A DOI 10. 11944 /j. issn. 1000-0518. 2015. 11. 150108 1000-0518
Electronic Supplementary Information
 Electronic Supplementary Information Unprecedented Carbon-Carbon Bond Cleavage in Nucleophilic Aziridine Ring Opening Reaction, Efficient Ring Transformation of Aziridines to Imidazolidin-4-ones Jin-Yuan
Electronic Supplementary Information Unprecedented Carbon-Carbon Bond Cleavage in Nucleophilic Aziridine Ring Opening Reaction, Efficient Ring Transformation of Aziridines to Imidazolidin-4-ones Jin-Yuan
Copper-Catalyzed Oxidative Dehydrogenative N-N Bond. Formation for the Synthesis of N,N -Diarylindazol-3-ones
 Electronic Supplementary Material (ESI) for Organic Chemistry Frontiers. This journal is the Partner Organisations 2016 Supporting information Copper-Catalyzed Oxidative Dehydrogenative - Bond Formation
Electronic Supplementary Material (ESI) for Organic Chemistry Frontiers. This journal is the Partner Organisations 2016 Supporting information Copper-Catalyzed Oxidative Dehydrogenative - Bond Formation
Supporting Information
 Supporting Information for AgOTf-catalyzed one-pot reactions of 2-alkynylbenzaldoximes with α,β-unsaturated carbonyl compounds Qiuping Ding 1, Dan Wang 1, Puying Luo* 2, Meiling Liu 1, Shouzhi Pu* 3 and
Supporting Information for AgOTf-catalyzed one-pot reactions of 2-alkynylbenzaldoximes with α,β-unsaturated carbonyl compounds Qiuping Ding 1, Dan Wang 1, Puying Luo* 2, Meiling Liu 1, Shouzhi Pu* 3 and
Metal-free Oxidative Coupling of Amines with Sodium Sulfinates: A Mild Access to Sulfonamides
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting information for Metal-free Oxidative Coupling of Amines with Sodium Sulfinates:
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting information for Metal-free Oxidative Coupling of Amines with Sodium Sulfinates:
Novel and Selective Palladium-Catalyzed Annulation of 2-Alkynylphenols to Form 2-Substituted 3-Halobenzo[b]furans. Supporting Information
![Novel and Selective Palladium-Catalyzed Annulation of 2-Alkynylphenols to Form 2-Substituted 3-Halobenzo[b]furans. Supporting Information Novel and Selective Palladium-Catalyzed Annulation of 2-Alkynylphenols to Form 2-Substituted 3-Halobenzo[b]furans. Supporting Information](/thumbs/64/50937700.jpg) Novel and Selective Palladium-Catalyzed Annulation of 2-Alkynylphenols to Form 2-Substituted 3-Halobenzo[b]furans Liang Yun, Shi Tang, Xu-Dong Zhang, Li-Qiu Mao, Ye-Xiang Xie and Jin-Heng Li* Key Laboratory
Novel and Selective Palladium-Catalyzed Annulation of 2-Alkynylphenols to Form 2-Substituted 3-Halobenzo[b]furans Liang Yun, Shi Tang, Xu-Dong Zhang, Li-Qiu Mao, Ye-Xiang Xie and Jin-Heng Li* Key Laboratory
Free Radical Initiated Coupling Reaction of Alcohols and. Alkynes: not C-O but C-C Bond Formation. Context. General information 2. Typical procedure 2
 Free Radical Initiated Coupling Reaction of Alcohols and Alkynes: not C-O but C-C Bond Formation Zhongquan Liu,* Liang Sun, Jianguo Wang, Jie Han, Yankai Zhao, Bo Zhou Institute of Organic Chemistry, Gannan
Free Radical Initiated Coupling Reaction of Alcohols and Alkynes: not C-O but C-C Bond Formation Zhongquan Liu,* Liang Sun, Jianguo Wang, Jie Han, Yankai Zhao, Bo Zhou Institute of Organic Chemistry, Gannan
Supporting Information
 Supporting Information Copper/Silver Cocatalyzed Oxidative Coupling of Vinylarenes with ICH 2 CF 3 or ICH 2 CHF 2 Leading to β-cf 3 /CHF 2 -Substituted Ketones Niannian Yi, Hao Zhang, Chonghui Xu, Wei
Supporting Information Copper/Silver Cocatalyzed Oxidative Coupling of Vinylarenes with ICH 2 CF 3 or ICH 2 CHF 2 Leading to β-cf 3 /CHF 2 -Substituted Ketones Niannian Yi, Hao Zhang, Chonghui Xu, Wei
and Selective Allylic Reduction of Allylic Alcohols and Their Derivatives with Benzyl Alcohol
 FeCl 3 6H 2 O-Catalyzed Disproportionation of Allylic Alcohols and Selective Allylic Reduction of Allylic Alcohols and Their Derivatives with Benzyl Alcohol Jialiang Wang, Wen Huang, Zhengxing Zhang, Xu
FeCl 3 6H 2 O-Catalyzed Disproportionation of Allylic Alcohols and Selective Allylic Reduction of Allylic Alcohols and Their Derivatives with Benzyl Alcohol Jialiang Wang, Wen Huang, Zhengxing Zhang, Xu
Rhodium-Catalyzed Oxidative Decarbonylative Heck-type Coupling of Aromatic Aldehydes with Terminal Alkenes
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Rhodium-Catalyzed Oxidative Decarbonylative Heck-type
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Rhodium-Catalyzed Oxidative Decarbonylative Heck-type
1.6 Other Intramolecular Decarboxylative Coupling Reactions Decarboxylative Coupling Reaction of Allyl Carboxylates
 Contents Part I New Carbon Carbon Coupling Reactions Based on Decarboxylation 1 Transition Metal-Catalyzed Decarboxylation and Decarboxylative Cross-Couplings... 3 1.1 Introduction... 3 1.2 Metal-Catalyzed
Contents Part I New Carbon Carbon Coupling Reactions Based on Decarboxylation 1 Transition Metal-Catalyzed Decarboxylation and Decarboxylative Cross-Couplings... 3 1.1 Introduction... 3 1.2 Metal-Catalyzed
Supplementary Figure S1. Single X-ray structure 3a at probability ellipsoids of 20%.
 Supplementary Figure S1. Single X-ray structure 3a at probability ellipsoids of 20%. S1 Supplementary Figure S2. Single X-ray structure 5a at probability ellipsoids of 20%. S2 H 15 Ph Ac Ac I AcH Ph Ac
Supplementary Figure S1. Single X-ray structure 3a at probability ellipsoids of 20%. S1 Supplementary Figure S2. Single X-ray structure 5a at probability ellipsoids of 20%. S2 H 15 Ph Ac Ac I AcH Ph Ac
Supporting information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 Supporting information Copper-catalysed intramolecular O-arylation: a simple
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 Supporting information Copper-catalysed intramolecular O-arylation: a simple
Rh(III)-Catalyzed C-H Amidation with N-hydroxycarbamates: A. new Entry to N-Carbamate Protected Arylamines
 Rh(III)-Catalyzed C-H Amidation with N-hydroxycarbamates: A new Entry to N-Carbamate Protected Arylamines Bing Zhou,* Juanjuan Du, Yaxi Yang,* Huijin Feng, Yuanchao Li Shanghai Institute of Materia Medica,
Rh(III)-Catalyzed C-H Amidation with N-hydroxycarbamates: A new Entry to N-Carbamate Protected Arylamines Bing Zhou,* Juanjuan Du, Yaxi Yang,* Huijin Feng, Yuanchao Li Shanghai Institute of Materia Medica,
Facile construction of the functionalized 4H-chromene via tandem. benzylation and cyclization. Jinmin Fan and Zhiyong Wang*
 Facile construction of the functionalized 4H-chromene via tandem benzylation and cyclization Jinmin Fan and Zhiyong Wang* Hefei National Laboratory for Physical Science at Microscale, Joint- Lab of Green
Facile construction of the functionalized 4H-chromene via tandem benzylation and cyclization Jinmin Fan and Zhiyong Wang* Hefei National Laboratory for Physical Science at Microscale, Joint- Lab of Green
Supporting Information. Asymmetric Binary-acid Catalysis with Chiral. Phosphoric Acid and MgF 2 : Catalytic
 Supporting Information Asymmetric Binary-acid Catalysis with Chiral Phosphoric Acid and MgF 2 : Catalytic Enantioselective Friedel-Crafts Reactions of β,γ- Unsaturated-α-Ketoesters Jian Lv, Xin Li, Long
Supporting Information Asymmetric Binary-acid Catalysis with Chiral Phosphoric Acid and MgF 2 : Catalytic Enantioselective Friedel-Crafts Reactions of β,γ- Unsaturated-α-Ketoesters Jian Lv, Xin Li, Long
College of Life Science, Dalian Nationalities University, Dalian , PR China.
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 Postsynthetic modification
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 Postsynthetic modification
D-Glucosamine-derived copper catalyst for Ullmann-type C- N coupling reaction: theoretical and experimental study
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 D-Glucosamine-derived copper catalyst for Ullmann-type C- N coupling reaction: theoretical
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 D-Glucosamine-derived copper catalyst for Ullmann-type C- N coupling reaction: theoretical
Vol. 41 No Journal of Jiangxi Normal University Natural Science Mar. 2017
 41 2 Vol 41 No 2 2017 3 Journal of Jiangxi Normal UniversityNatural Science Mar 2017 1000-5862201702-0145-05 * * 570228 α- 3 1 H NMR 13 C NMR O 621 3 A DOI10 16357 /j cnki issn1000-5862 2017 02 07 0 1
41 2 Vol 41 No 2 2017 3 Journal of Jiangxi Normal UniversityNatural Science Mar 2017 1000-5862201702-0145-05 * * 570228 α- 3 1 H NMR 13 C NMR O 621 3 A DOI10 16357 /j cnki issn1000-5862 2017 02 07 0 1
Supporting Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Silver or Cerium-Promoted Free Radical Cascade Difunctionalization
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Silver or Cerium-Promoted Free Radical Cascade Difunctionalization
Supporting Information
 Supporting Information Lewis acid catalyzed ring-opening reactions of methylenecyclopropanes with diphenylphosphine oxide in the presence of sulfur or selenium Min Shi,* Min Jiang and Le-Ping Liu State
Supporting Information Lewis acid catalyzed ring-opening reactions of methylenecyclopropanes with diphenylphosphine oxide in the presence of sulfur or selenium Min Shi,* Min Jiang and Le-Ping Liu State
9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer catalysts
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer
Protease-catalysed Direct Asymmetric Mannich Reaction in Organic Solvent
 Supplementary information for the paper Protease-catalysed Direct Asymmetric Mannich Reaction in Organic Solvent Yang Xue, Ling-Po Li, Yan-Hong He * & Zhi Guan * School of Chemistry and Chemical Engineering,
Supplementary information for the paper Protease-catalysed Direct Asymmetric Mannich Reaction in Organic Solvent Yang Xue, Ling-Po Li, Yan-Hong He * & Zhi Guan * School of Chemistry and Chemical Engineering,
SUPPORTING INFORMATION. Polystyrene-immobilized DABCO as a highly efficient and recyclable organocatalyst for Knoevenagel condensation
 Electronic Supplementary Material (ESI) for RSC Advances This journal is The Royal Society of Chemistry 213 SUPPORTING INFORMATION Polystyrene-immobilized DABCO as a highly efficient and recyclable organocatalyst
Electronic Supplementary Material (ESI) for RSC Advances This journal is The Royal Society of Chemistry 213 SUPPORTING INFORMATION Polystyrene-immobilized DABCO as a highly efficient and recyclable organocatalyst
Supporting Information
 Supporting Information for Lewis acid-catalyzed redox-neutral amination of 2-(3-pyrroline-1-yl)benzaldehydes via intramolecular [1,5]-hydride shift/isomerization reaction Chun-Huan Jiang, Xiantao Lei,
Supporting Information for Lewis acid-catalyzed redox-neutral amination of 2-(3-pyrroline-1-yl)benzaldehydes via intramolecular [1,5]-hydride shift/isomerization reaction Chun-Huan Jiang, Xiantao Lei,
A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
Lewis Acid Catalyzed Propargylation of Arenes with O-Propargyl Trichloroacetimidate: Synthesis of 1,3-Diarylpropynes
 Supporting Information for Lewis Acid Catalyzed Propargylation of Arenes with O-Propargyl Trichloroacetimidate: Synthesis of 1,3-Diarylpropynes Changkun Li and Jianbo Wang* Beijing National Laboratory
Supporting Information for Lewis Acid Catalyzed Propargylation of Arenes with O-Propargyl Trichloroacetimidate: Synthesis of 1,3-Diarylpropynes Changkun Li and Jianbo Wang* Beijing National Laboratory
gem-dichloroalkenes for the Construction of 3-Arylchromones
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Pd(OAc)2/S=PPh3 Accelerated Activation of gem-dichloroalkenes for the Construction of 3-Arylchromones
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Pd(OAc)2/S=PPh3 Accelerated Activation of gem-dichloroalkenes for the Construction of 3-Arylchromones
Supporting Information One-Pot Approach to Chiral Chromenes via Enantioselective Organocatalytic Domino Oxa-Michael-Aldol Reaction
 Supporting Information ne-pot Approach to Chiral Chromenes via Enantioselective rganocatalytic Domino xa-michael-aldol Reaction Hao Li, Jian Wang, Timiyin E-Nunu, Liansuo Zu, Wei Jiang, Shaohua Wei, *
Supporting Information ne-pot Approach to Chiral Chromenes via Enantioselective rganocatalytic Domino xa-michael-aldol Reaction Hao Li, Jian Wang, Timiyin E-Nunu, Liansuo Zu, Wei Jiang, Shaohua Wei, *
Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic acids with the assistance of isocyanide
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic
ESI for. A simple and efficient protocol for the palladium-catalyzed. ligand-free Suzuki reaction at room temperature in aqueous DMF.
 ESI for A simple and efficient protocol for the palladium-catalyzed ligand-free Suzuki reaction at room temperature in aqueous DMF Chun Liu,* Qijian i, Fanying Bao and Jieshan Qiu State Key Laboratory
ESI for A simple and efficient protocol for the palladium-catalyzed ligand-free Suzuki reaction at room temperature in aqueous DMF Chun Liu,* Qijian i, Fanying Bao and Jieshan Qiu State Key Laboratory
Supporting Information
 Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Ligand-Free Pd-Catalyzed Highly Selective Arylation of Allylic Esters with Retention of the Traditional Leaving Group (Supporting Information)
Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Ligand-Free Pd-Catalyzed Highly Selective Arylation of Allylic Esters with Retention of the Traditional Leaving Group (Supporting Information)
Cu-Catalyzed/Mediated Synthesis of N-Fluoroalkylanilines from Arylboronic Acids: Fluorine Effect on the Reactivity of Fluoroalkylamines
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 S1 Cu-Catalyzed/Mediated Synthesis of N-Fluoroalkylanilines from Arylboronic
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 S1 Cu-Catalyzed/Mediated Synthesis of N-Fluoroalkylanilines from Arylboronic
Site-Selective Suzuki-Miyaura Cross-Coupling Reactions of 2,3,4,5-Tetrabromofuran
 1 Site-Selective Suzuki-Miyaura Cross-Coupling Reactions of 2,3,4,5-Tetrabromofuran Munawar Hussain, a Rasheed Ahmad Khera, a Nguyen Thai Hung, a Peter Langer* a,b a Institut für Chemie, Universität Rostock,
1 Site-Selective Suzuki-Miyaura Cross-Coupling Reactions of 2,3,4,5-Tetrabromofuran Munawar Hussain, a Rasheed Ahmad Khera, a Nguyen Thai Hung, a Peter Langer* a,b a Institut für Chemie, Universität Rostock,
Copper-Catalyzed Oxidative Coupling of Acids with Alkanes Involving Dehydrogenation: Facile Access to Allylic Esters and Alkylalkenes
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supplementary information Copper-Catalyzed xidative Coupling of Acids with Alkanes Involving Dehydrogenation:
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supplementary information Copper-Catalyzed xidative Coupling of Acids with Alkanes Involving Dehydrogenation:
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Synthesis of 3-omosubstituted Pyrroles via Palladium- Catalyzed Intermolecular Oxidative Cyclization
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Synthesis of 3-omosubstituted Pyrroles via Palladium- Catalyzed Intermolecular Oxidative Cyclization
Room Temperature Highly Diastereoselective Zn-Mediated. Allylation of Chiral N-tert-Butanesulfinyl Imines: Remarkable Reaction Condition Controlled
 Supporting Information for: Room Temperature Highly Diastereoselective Zn-Mediated Allylation of Chiral N-tert-Butanesulfinyl Imines: Remarkable Reaction Condition Controlled Stereoselectivity Reversal
Supporting Information for: Room Temperature Highly Diastereoselective Zn-Mediated Allylation of Chiral N-tert-Butanesulfinyl Imines: Remarkable Reaction Condition Controlled Stereoselectivity Reversal
Supporting Information for Iron-catalyzed decarboxylative alkenylation of cycloalkanes with arylvinylic carboxylic acids via a radical process
 Supporting Information for Iron-catalyzed decarboxylative alkenylation of cycloalkanes with arylvinylic carboxylic acids via a radical process Jincan Zhao 1, Hong Fang 1, Jianlin Han* 1,2 and Yi Pan* 1
Supporting Information for Iron-catalyzed decarboxylative alkenylation of cycloalkanes with arylvinylic carboxylic acids via a radical process Jincan Zhao 1, Hong Fang 1, Jianlin Han* 1,2 and Yi Pan* 1
Supplementary material
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 Supplementary material Recyclable
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 Supplementary material Recyclable
Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3
 Supporting Information Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3 Shohei Shimokawa, Yuhsuke Kawagoe, Katsuhiko Moriyama, Hideo Togo* Graduate
Supporting Information Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3 Shohei Shimokawa, Yuhsuke Kawagoe, Katsuhiko Moriyama, Hideo Togo* Graduate
Vilsmeier Haack reagent-promoted formyloxylation of α-chloro-narylacetamides
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 205 Vilsmeier aack reagent-promoted formyloxylation of α-chloro-arylacetamides by formamide Jiann-Jyh
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 205 Vilsmeier aack reagent-promoted formyloxylation of α-chloro-arylacetamides by formamide Jiann-Jyh
Supporting Information
 Supporting Information 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Convenient and General Zinc-Catalyzed Borylation of Aryl Diazonium Salts and Aryltriazenes under Mild Conditions
Supporting Information 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Convenient and General Zinc-Catalyzed Borylation of Aryl Diazonium Salts and Aryltriazenes under Mild Conditions
Supporting Information
 Supporting Information Ceric Ammonium Nitrate (CAN) catalyzed efficient one-pot three component aza-diels-alder reactions for a facile synthesis of tetrahydropyranoquinoline derivatives Ravinder Goud Puligoundla
Supporting Information Ceric Ammonium Nitrate (CAN) catalyzed efficient one-pot three component aza-diels-alder reactions for a facile synthesis of tetrahydropyranoquinoline derivatives Ravinder Goud Puligoundla
Studies on Synthesis and Biological Activities of 2( 1 H21,2,42 Triazol212yl)2 2Arylthioethyl Substituted Phenyl Ketones
 2002 60 7, 1303 1310 ACTA CHIMICA SINICA Vol. 60, 2002 No. 7, 1303 1310 2( 1 H21,2,42 212 )2 2 ( 300071) Ξ Ξ 22(1 H21,2,42 212 )222 212 (2) 1,42, 3,,. R 1, R 1 = (CH 3 ) 3 C, R 1 = Ar, Ar., 1,42,, Studies
2002 60 7, 1303 1310 ACTA CHIMICA SINICA Vol. 60, 2002 No. 7, 1303 1310 2( 1 H21,2,42 212 )2 2 ( 300071) Ξ Ξ 22(1 H21,2,42 212 )222 212 (2) 1,42, 3,,. R 1, R 1 = (CH 3 ) 3 C, R 1 = Ar, Ar., 1,42,, Studies
Supporting Information for
 Supporting Information for An atom-economic route to densely functionalized thiophenes via base-catalyzed rearrangement of 5-propargyl-2H-thiopyran-4(3H)-ones Chunlin Tang a, Jian Qin b, Xingqi Li *a a
Supporting Information for An atom-economic route to densely functionalized thiophenes via base-catalyzed rearrangement of 5-propargyl-2H-thiopyran-4(3H)-ones Chunlin Tang a, Jian Qin b, Xingqi Li *a a
using metal-organic framework Cu-MOF-74 as an efficient heterogeneous catalyst Hanh T. H. Nguyen, Oanh T. K. Nguyen, Thanh Truong *, Nam T. S.
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Synthesis of imidazo[1,5-a]pyridines via oxidative amination of C(sp 3 )-H bond under air using
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Synthesis of imidazo[1,5-a]pyridines via oxidative amination of C(sp 3 )-H bond under air using
Asymmetric Transfer Hydrogenation of Ketones Catalyzed by Chiral Carbonyl Iron Systems
 2004 62 18, 1745 1750 ACTA CHIMICA SINICA Vol 62, 2004 No 18, 1745 1750 Ξ Ξ ( 361005) [ Et 3 NH] + [ HFe 3 (CO) 11 ] - 1,12, 98 % Fe 3 (CO) 12, Asymmetric Transfer Hydrogenation of Ketones Catalyzed by
2004 62 18, 1745 1750 ACTA CHIMICA SINICA Vol 62, 2004 No 18, 1745 1750 Ξ Ξ ( 361005) [ Et 3 NH] + [ HFe 3 (CO) 11 ] - 1,12, 98 % Fe 3 (CO) 12, Asymmetric Transfer Hydrogenation of Ketones Catalyzed by
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 A new route to enantiopure b-aryl-substituted b-amino acids and 4-aryl-substituted b-lactams through lipase-catalyzed
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 A new route to enantiopure b-aryl-substituted b-amino acids and 4-aryl-substituted b-lactams through lipase-catalyzed
Pd Catalyzed Carbonylation for the Construction of Tertiary and
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 Pd Catalyzed Carbonylation for the Construction of Tertiary and Quaternary
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 Pd Catalyzed Carbonylation for the Construction of Tertiary and Quaternary
Supporting Information
 Supporting Information Montmorillonite KSF-Catalyzed One-pot, Three-component, Aza-Diels- Alder Reactions of Methylenecyclopropanes With Arylaldehydes and Aromatic Amines Li-Xiong Shao and Min Shi* General
Supporting Information Montmorillonite KSF-Catalyzed One-pot, Three-component, Aza-Diels- Alder Reactions of Methylenecyclopropanes With Arylaldehydes and Aromatic Amines Li-Xiong Shao and Min Shi* General
Phosphorus Oxychloride as an Efficient Coupling Reagent for the Synthesis of Ester, Amide and Peptide under Mild Conditions
 Supplementary Information for Phosphorus xychloride as an Efficient Coupling Reagent for the Synthesis of Ester, Amide and Peptide under Mild Conditions u Chen,* a,b Xunfu Xu, a Liu Liu, a Guo Tang,* a
Supplementary Information for Phosphorus xychloride as an Efficient Coupling Reagent for the Synthesis of Ester, Amide and Peptide under Mild Conditions u Chen,* a,b Xunfu Xu, a Liu Liu, a Guo Tang,* a
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting Information 1. General experimental methods (S2). 2. Table 1: Initial studies (S2-S4).
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting Information 1. General experimental methods (S2). 2. Table 1: Initial studies (S2-S4).
SUPPORTING INFORMATION
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 SUPPORTIG IFORMATIO Visible light-mediated decarboxylative amination of indoline-2- carboxylic
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 SUPPORTIG IFORMATIO Visible light-mediated decarboxylative amination of indoline-2- carboxylic
Supporting Information
 Electronic upplementary Material (EI) for Green Chemistry. This journal is The Royal ociety of Chemistry 204 upporting Information ynthesis of sulfonamides via I 2 -mediated reaction of sodium sulfinates
Electronic upplementary Material (EI) for Green Chemistry. This journal is The Royal ociety of Chemistry 204 upporting Information ynthesis of sulfonamides via I 2 -mediated reaction of sodium sulfinates
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Modular Synthesis of Propargylamine Modified Cyclodextrins by a Gold(III)-catalyzed Three Component
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Modular Synthesis of Propargylamine Modified Cyclodextrins by a Gold(III)-catalyzed Three Component
multicomponent synthesis of 5-amino-4-
 Supporting Informartion for Molecular iodine-catalyzed one-pot multicomponent synthesis of 5-amino-4- (arylselanyl)-1h-pyrazoles Camila S. Pires 1, Daniela H. de Oliveira 1, Maria R. B. Pontel 1, Jean
Supporting Informartion for Molecular iodine-catalyzed one-pot multicomponent synthesis of 5-amino-4- (arylselanyl)-1h-pyrazoles Camila S. Pires 1, Daniela H. de Oliveira 1, Maria R. B. Pontel 1, Jean
phase: synthesis of biaryls, terphenyls and polyaryls
 Supporting Information for Studies on Pd/NiFe 2 O 4 catalyzed ligand-free Suzuki reaction in aqueous phase: synthesis of biaryls, terphenyls and polyaryls Sanjay R. Borhade and Suresh B. Waghmode* Address:
Supporting Information for Studies on Pd/NiFe 2 O 4 catalyzed ligand-free Suzuki reaction in aqueous phase: synthesis of biaryls, terphenyls and polyaryls Sanjay R. Borhade and Suresh B. Waghmode* Address:
Selective mono reduction of bisphosphine
 Griffith Research Online https://research-repository.griffith.edu.au Selective mono reduction of bisphosphine oxides under mild conditions Author etersson, Maria, Loughlin, Wendy, Jenkins, Ian ublished
Griffith Research Online https://research-repository.griffith.edu.au Selective mono reduction of bisphosphine oxides under mild conditions Author etersson, Maria, Loughlin, Wendy, Jenkins, Ian ublished
Supporting Information for
 Supporting Information for Palladium-Catalyzed C-H Bond Functionalization of C6-Arylpurines Hai-Ming Guo,* Wei-Hao Rao, Hong-Ying iu, Li-Li Jiang, Ge ng, Jia-Jia Jin, Xi-ing Yang, and Gui-Rong Qu* College
Supporting Information for Palladium-Catalyzed C-H Bond Functionalization of C6-Arylpurines Hai-Ming Guo,* Wei-Hao Rao, Hong-Ying iu, Li-Li Jiang, Ge ng, Jia-Jia Jin, Xi-ing Yang, and Gui-Rong Qu* College
Supporting Information. Experimental section
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Direct Palladium-Catalyzed Arylations of Aryl Bromides. with 2/9-Substituted Pyrimido[5,4-b]indolizines
![Direct Palladium-Catalyzed Arylations of Aryl Bromides. with 2/9-Substituted Pyrimido[5,4-b]indolizines Direct Palladium-Catalyzed Arylations of Aryl Bromides. with 2/9-Substituted Pyrimido[5,4-b]indolizines](/thumbs/87/95187440.jpg) Direct Palladium-Catalyzed Arylations of Aryl Bromides with 2/9-Substituted Pyrimido[5,4-b]indolizines Min Jiang, Ting Li, Linghua Meng, Chunhao Yang,* Yuyuan Xie*, and Jian Ding State Key Laboratory of
Direct Palladium-Catalyzed Arylations of Aryl Bromides with 2/9-Substituted Pyrimido[5,4-b]indolizines Min Jiang, Ting Li, Linghua Meng, Chunhao Yang,* Yuyuan Xie*, and Jian Ding State Key Laboratory of
Supplementary Data. Engineering, Nanjing University, Nanjing , P. R. China;
 Supplementary Data Synthesis, Chemo-selective Properties of Substituted 9-Aryl-9H-fluorenes from Triarylcarbinols and Enantiomerical Kinetics of Chiral 9-Methoxy-11-(naphthalen-1-yl)-11H-benzo[a]fluorene
Supplementary Data Synthesis, Chemo-selective Properties of Substituted 9-Aryl-9H-fluorenes from Triarylcarbinols and Enantiomerical Kinetics of Chiral 9-Methoxy-11-(naphthalen-1-yl)-11H-benzo[a]fluorene
Fluorinative Ring-opening of Cyclopropanes by Hypervalent Iodine Reagents. An Efficient Method for 1,3- Oxyfluorination and 1,3-Difluorination
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Supporting Information Fluorinative Ring-opening of Cyclopropanes by Hypervalent Iodine
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Supporting Information Fluorinative Ring-opening of Cyclopropanes by Hypervalent Iodine
Supporting Information
 Supporting Information Metal-Free Direct Intramolecular Carbotrifluoromethylation of Alkenes to Functionalized Trifluoromethyl Azaheterocycles Lei Li, Min Deng, Sheng-Cai Zheng, Ya-Ping Xiong, Li-Jiao
Supporting Information Metal-Free Direct Intramolecular Carbotrifluoromethylation of Alkenes to Functionalized Trifluoromethyl Azaheterocycles Lei Li, Min Deng, Sheng-Cai Zheng, Ya-Ping Xiong, Li-Jiao
Iodine-catalyzed synthesis of sulfur-bridged enaminones and chromones via double C(sp 2 )-H thiolation
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Iodine-catalyzed synthesis of sulfur-bridged enaminones and chromones via
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Iodine-catalyzed synthesis of sulfur-bridged enaminones and chromones via
Enantioselective Organocatalytic Michael Addition of Isorhodanines. to α, β-unsaturated Aldehydes
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 1 A Facile Way to Synthesize 2H-Chromenes: Reconsideration of the Reaction Mechanism between Salicylic Aldehyde and
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 1 A Facile Way to Synthesize 2H-Chromenes: Reconsideration of the Reaction Mechanism between Salicylic Aldehyde and
The Free Internet Journal for Organic Chemistry
 The Free Internet Journal for Organic Chemistry Paper Archive for Organic Chemistry Arkivoc 2018, part iii, S1-S6 Synthesis of dihydropyranones and dihydropyrano[2,3- d][1,3]dioxine-diones by cyclization
The Free Internet Journal for Organic Chemistry Paper Archive for Organic Chemistry Arkivoc 2018, part iii, S1-S6 Synthesis of dihydropyranones and dihydropyrano[2,3- d][1,3]dioxine-diones by cyclization
BINOL. Vol. 41 No Journal of Jiangxi Normal University Natural Science Sep C C
 41 5 Vol. 41 No. 5 2017 9 Journal of Jiangxi Normal University Natural Science Sep. 2017 1000-5862 2017 05-0495-07 BINOL α-ewg * 550001 BINOL α-ewg. BINOL 15% α-ewg α-ewg. BINOL O 626 A DOI 10. 16357 /j.
41 5 Vol. 41 No. 5 2017 9 Journal of Jiangxi Normal University Natural Science Sep. 2017 1000-5862 2017 05-0495-07 BINOL α-ewg * 550001 BINOL α-ewg. BINOL 15% α-ewg α-ewg. BINOL O 626 A DOI 10. 16357 /j.
Supporting Information
 Supporting Information An Approach to 3,6-Disubstituted 2,5-Dioxybenzoquinones via Two Sequential Suzuki Couplings. Three-step Synthesis of Leucomelone Xianwen Gan, Wei Jiang, Wei Wang,,,* Lihong Hu,,*
Supporting Information An Approach to 3,6-Disubstituted 2,5-Dioxybenzoquinones via Two Sequential Suzuki Couplings. Three-step Synthesis of Leucomelone Xianwen Gan, Wei Jiang, Wei Wang,,,* Lihong Hu,,*
Supplementary Material
 Supplementary Material Control experiments S2 Characterization data for the products S2-S7 References S8 MR spectra for the products S9-S28 S1 Control experiments 2a (99.5 mg, 0.5 mmol), I 2 (50.8 mg,
Supplementary Material Control experiments S2 Characterization data for the products S2-S7 References S8 MR spectra for the products S9-S28 S1 Control experiments 2a (99.5 mg, 0.5 mmol), I 2 (50.8 mg,
Supporting Information. Experimental section
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Anhydrous solvents were transferred by
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Anhydrous solvents were transferred by
Supplementary Material (ESI) for Organic & Biomolecular Chemistry This journal is (c) The Royal Society of Chemistry 2008
 Palladium(0)-catalyzed direct cross-coupling reaction of allylic alcohols with aryland alkenylboronic acids Hirokazu Tsukamoto, Tomomi Uchiyama, Takamichi Suzuki and Yoshinori Kondo Graduate School of
Palladium(0)-catalyzed direct cross-coupling reaction of allylic alcohols with aryland alkenylboronic acids Hirokazu Tsukamoto, Tomomi Uchiyama, Takamichi Suzuki and Yoshinori Kondo Graduate School of
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2009
 Supporting Information Copyright Wiley-VC Verlag Gmb & Co. KGaA, 69451 Weinheim, 2009 Supporting Information for Graphite-Supported Gold anoparticles as Efficient Catalyst for Aerobic xidation of Benzylic
Supporting Information Copyright Wiley-VC Verlag Gmb & Co. KGaA, 69451 Weinheim, 2009 Supporting Information for Graphite-Supported Gold anoparticles as Efficient Catalyst for Aerobic xidation of Benzylic
Tributylphosphine-Catalyzed Cycloaddition of Aziridines with Carbon Disulfide and Isothiocyanate
 upporting Information Tributylphosphine-Catalyzed Cycloaddition of Aziridines with Carbon Disulfide and Isothiocyanate Jing-Yu Wu, Zhi-Bin Luo, Li-Xin Dai and Xue-Long Hou* a tate Key Laboratory of Organometallic
upporting Information Tributylphosphine-Catalyzed Cycloaddition of Aziridines with Carbon Disulfide and Isothiocyanate Jing-Yu Wu, Zhi-Bin Luo, Li-Xin Dai and Xue-Long Hou* a tate Key Laboratory of Organometallic
Synthesis of Imines from Amines in Aliphatic Alcohols on Pd/ZrO 2 Catalyst at Ambient Conditions
 This journal is The Royal Society of Chemistry 213 Synthesis of Imines from Amines in Aliphatic Alcohols on Pd/ZrO 2 Catalyst at Ambient Conditions Wenjing Cui, a Bao Zhaorigetu,* a Meilin Jia, a and Wulan
This journal is The Royal Society of Chemistry 213 Synthesis of Imines from Amines in Aliphatic Alcohols on Pd/ZrO 2 Catalyst at Ambient Conditions Wenjing Cui, a Bao Zhaorigetu,* a Meilin Jia, a and Wulan
Supporting Information
 Supporting Information Direct Olefination of Alcohols with Sulfones by Using Heterogeneous Platinum Catalysts S. M. A. Hakim Siddiki, [a] Abeda Sultana Touchy, [b] Kenichi Kon, [b] [a, b] and Ken-ichi
Supporting Information Direct Olefination of Alcohols with Sulfones by Using Heterogeneous Platinum Catalysts S. M. A. Hakim Siddiki, [a] Abeda Sultana Touchy, [b] Kenichi Kon, [b] [a, b] and Ken-ichi
Reusable Cu 2 O/PPh 3 /TBAB System for the Cross-Couplings of Aryl Halides and Heteroaryl Halides with Terminal Alkynes. Supporting Information
 Reusable Cu 2 O/PPh 3 /TBAB System for the Cross-Couplings of Aryl Halides and Heteroaryl Halides with Terminal Alkynes Bo-Xiao Tang, Feng Wang, Jin-Heng Li,* Ye-Xiang Xie, and Man-Bo Zhang* Key Laboratory
Reusable Cu 2 O/PPh 3 /TBAB System for the Cross-Couplings of Aryl Halides and Heteroaryl Halides with Terminal Alkynes Bo-Xiao Tang, Feng Wang, Jin-Heng Li,* Ye-Xiang Xie, and Man-Bo Zhang* Key Laboratory
Supporting information
 Electronic upplementary Material (EI) for New Journal of Chemistry. This journal is The Royal ociety of Chemistry and the Centre National de la Recherche cientifique 7 upporting information Lipase catalyzed,-addition
Electronic upplementary Material (EI) for New Journal of Chemistry. This journal is The Royal ociety of Chemistry and the Centre National de la Recherche cientifique 7 upporting information Lipase catalyzed,-addition
Regioselectivity in the Stille coupling reactions of 3,5- dibromo-2-pyrone.
 Regioselectivity in the Stille coupling reactions of 3,5- dibromo-2-pyrone. Won-Suk Kim, Hyung-Jin Kim and Cheon-Gyu Cho Department of Chemistry, Hanyang University, Seoul 133-791, Korea Experimental Section
Regioselectivity in the Stille coupling reactions of 3,5- dibromo-2-pyrone. Won-Suk Kim, Hyung-Jin Kim and Cheon-Gyu Cho Department of Chemistry, Hanyang University, Seoul 133-791, Korea Experimental Section
Highly enantioselective cascade synthesis of spiropyrazolones. Supporting Information. NMR spectra and HPLC traces
 Highly enantioselective cascade synthesis of spiropyrazolones Alex Zea a, Andrea-Nekane R. Alba a, Andrea Mazzanti b, Albert Moyano a and Ramon Rios a,c * Supporting Information NMR spectra and HPLC traces
Highly enantioselective cascade synthesis of spiropyrazolones Alex Zea a, Andrea-Nekane R. Alba a, Andrea Mazzanti b, Albert Moyano a and Ramon Rios a,c * Supporting Information NMR spectra and HPLC traces
LUO, Hong2Qun LIU, Shao2Pu Ξ LI, Nian2Bing
 2003 61 3, 435 439 ACTA CHIMICA SINICA Vol 61, 2003 No 3, 435 439 2 ΞΞ ( 400715), 2, 2, 2, 3/ 2 2,, 2,, Ne w Methods for the Determination of the Inclusion Constant between Procaine Hydrochloride and 2Cyclodextrin
2003 61 3, 435 439 ACTA CHIMICA SINICA Vol 61, 2003 No 3, 435 439 2 ΞΞ ( 400715), 2, 2, 2, 3/ 2 2,, 2,, Ne w Methods for the Determination of the Inclusion Constant between Procaine Hydrochloride and 2Cyclodextrin
Supporting Information
 Supporting Information Regioselective Reversal in the Cyclization of 2-Diazo-3,5-dioxo-6-ynoates (ynones, ynamide): Construction of -Pyrones and 3(2H)-Furanones Starting from Identical Materials Feng Wang,
Supporting Information Regioselective Reversal in the Cyclization of 2-Diazo-3,5-dioxo-6-ynoates (ynones, ynamide): Construction of -Pyrones and 3(2H)-Furanones Starting from Identical Materials Feng Wang,
Eco-friendly synthesis of diverse and valuable 2-pyridones by catalyst- and solvent-free thermal multicomponent domino reaction
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 SUPPRTIG IFRMATI Eco-friendly synthesis of diverse and valuable 2-pyridones by catalyst-
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 SUPPRTIG IFRMATI Eco-friendly synthesis of diverse and valuable 2-pyridones by catalyst-
Supporting Information for Synthesis of Fused N-Heterocycles via Tandem C-H Activation
 This journal is The Royal Society of Chemistry 212 Supporting Information for Synthesis of Fused -Heterocycles via Tandem C-H Activation Ge Meng, Hong-Ying iu, Gui-Rong Qu, John S. Fossey, Jian-Ping Li,*
This journal is The Royal Society of Chemistry 212 Supporting Information for Synthesis of Fused -Heterocycles via Tandem C-H Activation Ge Meng, Hong-Ying iu, Gui-Rong Qu, John S. Fossey, Jian-Ping Li,*
Copper-mediated radical cross-coupling reaction of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) with phenols or thiophenols. Support Information
 Copper-mediated radical cross-coupling reaction of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) with phenols or thiophenols Dr. Xiao un Tang and Prof. Qing un Chen* Key Laboratory of Organofluorine Chemistry,
Copper-mediated radical cross-coupling reaction of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) with phenols or thiophenols Dr. Xiao un Tang and Prof. Qing un Chen* Key Laboratory of Organofluorine Chemistry,
Supporting Information
 Supporting Information Synthesis and Electroactive Properties of Poly(amidoamine) Dendrimers with an Aniline Pentamer Shell Wei-I Hung a, Chih-Bing Hung a, Ya-Han Chang a, Jiun-Kuang Dai a, Yan Li b, Hai
Supporting Information Synthesis and Electroactive Properties of Poly(amidoamine) Dendrimers with an Aniline Pentamer Shell Wei-I Hung a, Chih-Bing Hung a, Ya-Han Chang a, Jiun-Kuang Dai a, Yan Li b, Hai
The N,S-Bidentate Ligand Assisted Pd-Catalyzed C(sp 2 )-H. Carbonylation using Langlois Reagent as CO Source. Supporting Information.
 Electronic upplementary Material (EI) for rganic & Biomolecular Chemistry. This journal is The Royal ociety of Chemistry 2018 The,-Bidentate Ligand Assisted Pd-Catalyzed C(sp 2 )-H Carbonylation using
Electronic upplementary Material (EI) for rganic & Biomolecular Chemistry. This journal is The Royal ociety of Chemistry 2018 The,-Bidentate Ligand Assisted Pd-Catalyzed C(sp 2 )-H Carbonylation using
Supporting Information
 Supporting Information Metal-catalyzed Stereoselective and Protecting-group-free Synthesis of 1,2-cis-Glycosides Using 4,6-Dimethoxy-1,3,5-triazin-2-yl Glycosides as Glycosyl Donors Tomonari Tanaka,* 1
Supporting Information Metal-catalyzed Stereoselective and Protecting-group-free Synthesis of 1,2-cis-Glycosides Using 4,6-Dimethoxy-1,3,5-triazin-2-yl Glycosides as Glycosyl Donors Tomonari Tanaka,* 1
Supporting information. Palladium nanoparticles generated in situ used as catalysts in carbonylative cross-coupling in aqueous medium
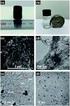 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Supporting information Palladium nanoparticles generated in situ used as catalysts in carbonylative
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Supporting information Palladium nanoparticles generated in situ used as catalysts in carbonylative
Supporting Information for. Rhodium-Catalyzed β-selective Oxidative Heck-Type
 Supporting Information for Rhodium-Catalyzed β-selective Oxidative Heck-Type Coupling of Vinyl Acetate via C-H Activation Hui-Jun Zhang,* Weidong Lin, Feng Su, and Ting-Bin Wen* Department of Chemistry,
Supporting Information for Rhodium-Catalyzed β-selective Oxidative Heck-Type Coupling of Vinyl Acetate via C-H Activation Hui-Jun Zhang,* Weidong Lin, Feng Su, and Ting-Bin Wen* Department of Chemistry,
-4 6- 许招会, 熊 Vol. 37 No Journal of Jiangxi Normal University Natural Science Jul Scheme 1.
 37 4 Vol 37 No 4 2013 7 Journal of Jiangxi Normal UniversityNatural Science Jul 2013 1000-5862201304-0337-05 5- -2 2- -1 3- -4 6- 许招会, 熊 斌, 雷志伟 330027 LaPW 12 O 40 2 2- -1 3- -4 6-8 5- -2 2- -1 3- -4 6-5%
37 4 Vol 37 No 4 2013 7 Journal of Jiangxi Normal UniversityNatural Science Jul 2013 1000-5862201304-0337-05 5- -2 2- -1 3- -4 6- 许招会, 熊 斌, 雷志伟 330027 LaPW 12 O 40 2 2- -1 3- -4 6-8 5- -2 2- -1 3- -4 6-5%
Study of Ne w Chemiluminescence Technique Recognition of Sodium Azide by External Reference Method
 2004 62 8, 794 798 ACTA CHIMICA SINICA Vol 62, 2004 No 8, 794 798 Ξ Ξ ( 200032),, : :H 2 O 2 2CH 3 CN2 ( ) H 2 O 2 2CH 3 CN2N2 252 ( ),,, IgG Study of Ne w Chemiluminescence Technique Recognition of Sodium
2004 62 8, 794 798 ACTA CHIMICA SINICA Vol 62, 2004 No 8, 794 798 Ξ Ξ ( 200032),, : :H 2 O 2 2CH 3 CN2 ( ) H 2 O 2 2CH 3 CN2N2 252 ( ),,, IgG Study of Ne w Chemiluminescence Technique Recognition of Sodium
Supporting Information. Microwave-assisted construction of triazole-linked amino acid - glucoside conjugates as novel PTP1B inhibitors
 Supporting Information Microwave-assisted construction of triazole-linked amino acid - glucoside conjugates as novel PTP1B inhibitors Xiao-Peng He, abd Cui Li, d Xiao-Ping Jin, b Zhuo Song, b Hai-Lin Zhang,
Supporting Information Microwave-assisted construction of triazole-linked amino acid - glucoside conjugates as novel PTP1B inhibitors Xiao-Peng He, abd Cui Li, d Xiao-Ping Jin, b Zhuo Song, b Hai-Lin Zhang,
Electronic Supplementary Information
 Electronic Supplementary Information NbCl 3 -catalyzed [2+2+2] intermolecular cycloaddition of alkynes and alkenes to 1,3-cyclohexadiene derivatives Yasushi Obora,* Keisuke Takeshita and Yasutaka Ishii*
Electronic Supplementary Information NbCl 3 -catalyzed [2+2+2] intermolecular cycloaddition of alkynes and alkenes to 1,3-cyclohexadiene derivatives Yasushi Obora,* Keisuke Takeshita and Yasutaka Ishii*
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry Electronic Supplementary Information (ESI) For Iron-Catalysed xidative Amidation of Alcohols with Amines Silvia Gaspa, a Andrea
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry Electronic Supplementary Information (ESI) For Iron-Catalysed xidative Amidation of Alcohols with Amines Silvia Gaspa, a Andrea
Jing-Yu Guo, Rui-Han Dai, Wen-Cong Xu, Ruo-Xin Wu and Shi-Kai Tian*
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 TfNHNHBoc as a SCF 3 source in the sulfenylation of indoles Jing-Yu Guo, Rui-Han Dai, Wen-Cong
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 TfNHNHBoc as a SCF 3 source in the sulfenylation of indoles Jing-Yu Guo, Rui-Han Dai, Wen-Cong
Available online at shd.org.rs/jscs/
 J. Serb. Chem. Soc. 78 (1) S1 S8 (2013) Supplementary material SUPPLEMENTARY MATERIAL TO Metal complexes of N'-[2-hydroxy-5-(phenyldiazenyl)- benzylidene]isonicotinohydrazide. Synthesis, spectroscopic
J. Serb. Chem. Soc. 78 (1) S1 S8 (2013) Supplementary material SUPPLEMENTARY MATERIAL TO Metal complexes of N'-[2-hydroxy-5-(phenyldiazenyl)- benzylidene]isonicotinohydrazide. Synthesis, spectroscopic
Supporting Information. for
 Supporting Information for A general synthetic route to [Cu(X)(NHC)] (NHC = N- heterocyclic carbene, X =Cl, Br, I) complexes Orlando Santoro, Alba Collado, Alexandra M. Z. Slawin, Steven P. Nolan and Catherine
Supporting Information for A general synthetic route to [Cu(X)(NHC)] (NHC = N- heterocyclic carbene, X =Cl, Br, I) complexes Orlando Santoro, Alba Collado, Alexandra M. Z. Slawin, Steven P. Nolan and Catherine
First DMAP-mediated direct conversion of Morita Baylis. Hillman alcohols into γ-ketoallylphosphonates: Synthesis of
 Supporting Information File 1 for First DMAP-mediated direct conversion of Morita Baylis Hillman alcohols into γ-ketoallylphosphonates: Synthesis of γ-aminoallylphosphonates Marwa Ayadi 1,2, Haitham Elleuch
Supporting Information File 1 for First DMAP-mediated direct conversion of Morita Baylis Hillman alcohols into γ-ketoallylphosphonates: Synthesis of γ-aminoallylphosphonates Marwa Ayadi 1,2, Haitham Elleuch
