F322. CHEMISTRY A Chains, Energy and Resources ADVANCED SUBSIDIARY GCE. Thursday 20 January 2011 Afternoon. Duration: 1 hour 45 minutes
|
|
|
- ŌἈβαδδών Καλλιγάς
- 8 χρόνια πριν
- Προβολές:
Transcript
1 ADVANCED SUBSIDIARY GCE CEMISTRY A Chains, Energy and Resources F322 *OCE/23212* Candidates answer on the question paper. OCR Supplied Materials: Data Sheet for Chemistry A (inserted) Other Materials Required: Scientific calculator Thursday 20 January 2011 Afternoon Duration: 1 hour 45 minutes * F * INSTRUCTIONS TO CANDIDATES The insert will be found in the centre of this document. Write your name, centre number and candidate number in the boxes above. Please write clearly and in capital letters. Use black ink. Pencil may be used for graphs and diagrams only. Read each question carefully. Make sure you know what you have to do before starting your answer. Write your answer to each question in the space provided. If additional space is required, you should use the lined pages at the end of this booklet. The question number(s) must be clearly shown. Answer all the questions. Do not write in the bar codes. INFORMATION FOR CANDIDATES The number of marks is given in brackets [ ] at the end of each question or part question. Where you see this icon you will be awarded marks for the quality of written communication in your answer. This means for example you should: ensure that text is legible and that spelling, punctuation and grammar are accurate so that meaning is clear; organise information clearly and coherently, using specialist vocabulary when appropriate. You may use a scientific calculator. A copy of the Data Sheet for Chemistry A is provided as an insert with this question paper. You are advised to show all the steps in any calculations. The total number of marks for this paper is 100. This document consists of 24 pages. Any blank pages are indicated. [/500/7834] DC (SJF5669/SW) 23212/4 OCR is an exempt Charity Turn over
2 2 BLANK PAGE PLEASE DO NOT WRITE ON TIS PAGE
3 3 Answer all the questions. 1 Crude oil is a source of alkanes. (a) Fractional distillation is used to separate useful hydrocarbons found in crude oil. Explain, in terms of intermolecular forces, how fractional distillation works.... [2] (b) The petroleum industry processes straight-chained alkanes into cycloalkanes such as cyclopentane and cyclohexane. 2 C 2 C 2 C 2 C cyclopentane cyclohexane (i) Deduce the general formula of a cycloalkane.... [1] (ii) Construct the equation to show the formation of cyclohexane from hexane. [1] (iii) Suggest why the petroleum industry processes hexane into cyclohexane.... [1] Turn over
4 4 (c) The flowchart below shows some of the organic compounds that could be made starting from cyclohexane. Cl 2 / UV Cl chlorocyclohexane KO(aq) / warm compound A K 2 Cr 2 O 7 (aq) 3 PO 4 / heat 2 SO 4 (aq) O cyclohexanol cyclohexene Br Br 2 compound B Br Br (i) Explain why cyclohexene is described as unsaturated and as a hydrocarbon.... [2] (ii) The reaction between chlorine and cyclohexane is an example of radical substitution. State one problem of using this reaction to prepare a sample of chlorocyclohexane.... [1]
5 (iii) 5 The formation of cyclohexanol from chlorocyclohexane involves the reaction of a nucleophile, the hydroxide ion. Suggest what feature of the hydroxide ion makes it able to act as a nucleophile.... [1] (iv) Using the flowchart, draw the structures of compound A and compound B. compound A compound B [2] (v) Describe, using the curly arrow model, the mechanism for the reaction between Br 2 and cyclohexene. Show relevant dipoles and charges. 2 C 2 C C Br C Br [4] [Total: 15] Turn over
6 2 Butyl ethanoate is an ester used as a flavouring. This ester can be synthesised from butan-1-ol by two different processes. Process 1 is a one-step process that involves a reversible reaction. 6 C 3 O + C 3 COO C 3 COO C O The percentage yield for process 1 is 67.1%. The atom economy for process 1 is 86.6%. Process 2 is a two-step process. C 3 COO + SOCl 2 C 3 COCl + SO 2 + Cl C 3 O + C 3 COCl C 3 COO C 3 + Cl The overall percentage yield for process 2 is 93.3%. The overall atom economy for process 2 is 45.8%. (a) Draw the skeletal formula for the ester butyl ethanoate. [1] (b) Show that the atom economy for process 1 is 86.6%. [2]
7 (c) A research chemist investigates process 1. She finds that 6.25 g of butan-1-ol forms 6.57 g of butyl ethanoate. 7 (i) Suggest the conditions needed for this reaction.... [2] (ii) Show that the percentage yield of process 1 is 67.1%. (d) Explain why process 2 has a high percentage yield but a low atom economy.... [2] (e) Suggest two reasons why butyl ethanoate is manufactured by process 1 rather than by process [2] [2] [Total: 11] Turn over
8 3 Enthalpy changes of reaction can be determined by experiment or by using bond enthalpies. (a) What is meant by the term enthalpy change of reaction? 8... [2] (b) Solid ammonium thiocyanate, N 4 SCN, reacts with solid barium hydroxide, Ba(O) 2, as shown in the equation below. 2N 4 SCN(s) + Ba(O) 2 (s) Ba(SCN) 2 (s) O(l) + 2N 3 (g) A research chemist carries out an experiment to determine the enthalpy change of this reaction. thermometer boiling tube 50.0g of water mixture of ammonium thiocyanate and barium hydroxide insulated beaker In the experiment, g of N 4 SCN is reacted with a slight excess of Ba(O) 2. The reaction absorbs energy, cooling the 50.0 g of water from 21.9 C to 10.9 C. (i) Calculate the energy absorbed, in kj, during this reaction. The specific heat capacity of water = 4.2 J g 1 K 1. energy =...kj [2]
9 (ii) 9 Calculate the amount, in moles, of N 4 SCN used by the research chemist. amount =... mol [1] (iii) Calculate the enthalpy change of reaction. Include the sign in your answer. Give your answer to two significant figures. Δ r =...kj mol 1 [3] PART (c) CONTINUES ON PAGE 10 Turn over
10 10 (c) Standard enthalpy changes of reaction can also be determined using average bond enthalpies. (i) What is meant by the term average bond enthalpy?... [2] Table 3.1 below shows some average bond enthalpies. bond average bond enthalpy / kj mol 1 C +415 C C +345 C=C +611 Table 3.1 (ii) Explain the bonding in a C=C double bond. Use the orbital overlap model.... [2] (iii) Suggest why the average bond enthalpy of a C=C bond is not twice the bond enthalpy of a C C bond.... [1]
11 (iv) 11 Propane can be cracked to make ethene. C C C C C + C Using the average bond enthalpies in Table 3.1, calculate the enthalpy change of this reaction. Δ r =...kj mol 1 [2] (v) The actual value for the enthalpy change of this reaction is +81 kj mol 1. Suggest a reason why the actual value for the enthalpy change of this reaction is different from the calculated value.... [1] [Total: 16] Turn over
12 4 Catalysts speed up the rate of a reaction without being consumed by the overall reaction. (a) Chlorine radicals in the stratosphere act as a catalyst for ozone depletion. 12 (i) Research chemists have proposed possible reaction mechanisms for ozone depletion. The equations below represent part of such a mechanism. Complete the equations. Cl + O 3 + ClO + + O 2 [2] (ii) Write an equation for the overall reaction in (i).... [1] (b) One of the catalysed reactions that takes place in a catalytic converter is shown below. 2CO(g) + 2NO(g) N 2 (g) + 2CO 2 (g) The catalyst used is platinum/rhodium attached to a ceramic surface. Outline the stages that take place in a catalytic converter to allow CO to react with NO.... [4]
13 13 (c) Explain, using an enthalpy profile diagram and a Boltzmann distribution, how the presence of a catalyst increases the rate of reaction. In your answer you should organise your answer and use the correct technical terms.... [7] Turn over
14 14 (d) Explain why many industrial manufacturing processes use catalysts. Include in your answer ideas about sustainability, economics and pollution control.... [4] [Total: 18]
15 15 5 This question is about halogenated hydrocarbons. (a) alogenoalkanes undergo nucleophilic substitution reactions with ammonia to form amines. Amines contain the N 2 functional group. For example, 1-bromopropane reacts with ammonia to form propylamine, C 3 N 2. C 3 Br + 2N 3 C 3 N 2 + N 4 Br (i) Iodoethane is reacted with ammonia. Write an equation for this reaction.... [2] (ii) The first step in the mechanism of the reaction between C 3 Br and N 3 is shown below. It is incomplete. + C 3 C Br C 3 C N Complete the mechanism. Include relevant dipoles, lone pairs, curly arrows and the missing product. [3] Turn over
16 (b) A student investigates the rate of hydrolysis of six halogenoalkanes. 16 The student mixes 5 cm 3 of ethanol with five drops of halogenoalkane. This mixture is warmed to 50 C in a water bath. The student adds 5 cm 3 of aqueous silver nitrate, also heated to 50 C, to the halogenoalkane. The time taken for a precipitate to form is recorded in a results table. The student repeats the whole experiment at 60 C instead of 50 C. time taken for a precipitate to form / s halogenoalkane at 50 C at 60 C C 3 Cl C 3 Br C 3 I C 3 CBrC (C 3 ) 2 C Br (C 3 ) 3 CBr Describe and explain the factors that affect the rate of hydrolysis of halogenoalkanes. Include ideas about the halogen in the halogenoalkanes the groups attached to the carbon of the carbon halogen bond (the type of halogenoalkane) the temperature of the hydrolysis. In your answer you should link the evidence with your explanation.
17 17... [7] (c) Poly(tetrafluoroethene), PTFE, and poly(chloroethene), PVC, are halogenated plastics. (i) Write an equation, using displayed formulae, for the reaction to form PTFE from its monomer. [3] (ii) The combustion of waste polymers can be used for energy production. What problem is caused by disposing of PTFE and PVC in this way?... [1] [Total: 16] Turn over
18 6 Mass spectrometry and infrared spectroscopy are used in analysis. 18 (a) The mass spectrum of compound Z is shown below relative abundance (%) m / z Compound Z has the molecular formula C 3 6 O x. (i) Using the mass spectrum, deduce the value of x in C 3 6 O x. Explain your answer.... [2] (ii) Suggest a possible structure for Z. (iii) Suggest the formula of an ion that gives rise to the peak at m/z = 29 in this spectrum.... [1] (b) A space probe has detected the presence of the element iron on the surface of the planet Mars. Outline how a mass spectrum would show the presence of iron. [1]... [1]
19 (c) The space probe also detected different isotopes of sulfur on Mars. 19 (i) Outline how the mass spectrum would show how many different isotopes of sulfur were present on Mars.... [1] (ii) The relative atomic mass of the sulfur found by the space probe was different from the relative atomic mass of sulfur on Earth. Suggest why.... [1] (d) An environmental chemist used infrared spectroscopy to monitor air pollution outside a petrol station. The infrared spectrum below was obtained from one of these pollutants transmittance (%) wavenumber / cm What evidence is there in the spectrum that the pollutant may be a hydrocarbon rather than an alcohol or a carbonyl compound?... [1] Turn over
20 20 (e) The infrared spectrum of a drug is shown below transmittance (%) wavenumber / cm 1 Suggest, with reasons, possible functional group(s) present in the drug.... [2] [Total: 10]
21 21 7 Biofuels such as bioethanol and biodiesel are increasingly being used as an alternative to fossil fuels to provide energy. (a) Describe, with the aid of an equation, how bioethanol is manufactured by fermentation.... [3] (b) Biodiesel is obtained from plant oils. The manufacture involves several stages, all of which have a high energy requirement. Biodiesel is often described as being carbon-neutral because: plants convert atmospheric carbon dioxide into carbon compounds on burning biodiesel this carbon dioxide is returned to the atmosphere. (i) Construct an equation to show the complete combustion of biodiesel. Assume that the molecular formula of the biodiesel is C O [2] (ii) Suggest why biodiesel is not completely carbon-neutral.... [1] (c) Many scientists suggest that society should use more biofuels rather than fossil fuels to provide energy. Other scientists are worried that biofuels will need large areas of land to grow suitable crops. Suggest disadvantages or advantages, other than being carbon-neutral, of using more biofuels.... [3] Turn over
22 22 (d) Unsaturated compounds in plant oils can also be used to make margarine. Describe how.... [2] (e) Part of the structure of an unsaturated compound in plant oils is shown below: C=C (i) Draw the displayed formula of the Z isomer of this part of the structure. [1] (ii) Explain why this part of the structure can have an E and a Z isomer.... [2] [Total: 14] END OF QUESTION PAPER
23 23 ADDITIONAL PAGE If additional space is required, you should use the lined pages below. The question number(s) must be clearly shown
24 24 ADDITIONAL PAGE Copyright Information OCR is committed to seeking permission to reproduce all third-party content that it uses in its assessment materials. OCR has attempted to identify and contact all copyright holders whose work is used in this paper. To avoid the issue of disclosure of answer-related information to candidates, all copyright acknowledgements are reproduced in the OCR Copyright Acknowledgements Booklet. This is produced for each series of examinations and is freely available to download from our public website ( after the live examination series. If OCR has unwittingly failed to correctly acknowledge or clear any third-party content in this assessment material, OCR will be happy to correct its mistake at the earliest possible opportunity. For queries or further information please contact the Copyright Team, First Floor, 9 ills Road, Cambridge CB2 1GE. OCR is part of the Cambridge Assessment Group; Cambridge Assessment is the brand name of University of Cambridge Local Examinations Syndicate (UCLES), which is itself a department of the University of Cambridge.
, are amongst the easiest enthalpy changes to determine directly.... [2]
![, are amongst the easiest enthalpy changes to determine directly.... [2] , are amongst the easiest enthalpy changes to determine directly.... [2]](/thumbs/76/73796917.jpg) 1 Enthalpy changes of combustion, Δ c, are amongst the easiest enthalpy changes to determine directly. (a) Define the term enthalpy change of combustion.. [2] (b) A student carried out an experiment to
1 Enthalpy changes of combustion, Δ c, are amongst the easiest enthalpy changes to determine directly. (a) Define the term enthalpy change of combustion.. [2] (b) A student carried out an experiment to
Mean bond enthalpy Standard enthalpy of formation Bond N H N N N N H O O O
 Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *4358398658* GREEK 0543/04 Paper 4 Writing May/June 2015 1 hour Candidates answer on the Question
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *4358398658* GREEK 0543/04 Paper 4 Writing May/June 2015 1 hour Candidates answer on the Question
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *2517291414* GREEK 0543/02 Paper 2 Reading and Directed Writing May/June 2013 1 hour 30 minutes
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *2517291414* GREEK 0543/02 Paper 2 Reading and Directed Writing May/June 2013 1 hour 30 minutes
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *3148288373* GREEK 0543/04 Paper 4 Writing May/June 2016 1 hour Candidates answer on the Question
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *3148288373* GREEK 0543/04 Paper 4 Writing May/June 2016 1 hour Candidates answer on the Question
Tuesday 13 June 2017 Afternoon
 Oxford Cambridge and RSA Tuesday 13 June 2017 Afternoon A2 GCE CHEMISTRY A F324/01 Rings, Polymers and Analysis *6765314342* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet
Oxford Cambridge and RSA Tuesday 13 June 2017 Afternoon A2 GCE CHEMISTRY A F324/01 Rings, Polymers and Analysis *6765314342* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *8175930111* GREEK 0543/04 Paper 4 Writing May/June 2017 1 hour Candidates answer on the Question
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *8175930111* GREEK 0543/04 Paper 4 Writing May/June 2017 1 hour Candidates answer on the Question
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *1880009435* GREEK 0543/04 Paper 4 Writing May/June 2018 1 hour Candidates answer on the Question
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *1880009435* GREEK 0543/04 Paper 4 Writing May/June 2018 1 hour Candidates answer on the Question
*2354431106* GREEK 0543/02 Paper 2 Reading and Directed Writing May/June 2009
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *2354431106* GREEK 0543/02 Paper 2 Reading and Directed Writing May/June 2009 1 hour 30 minutes
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *2354431106* GREEK 0543/02 Paper 2 Reading and Directed Writing May/June 2009 1 hour 30 minutes
Br, is used in the production of polymers.
 1 Allyl bromide, =CH Br, is used in the production of polymers. (a) Part of the C=C double bond in allyl bromide is called a π-bond. Draw a labelled diagram to show the formation of the π-bond. [2] (b)
1 Allyl bromide, =CH Br, is used in the production of polymers. (a) Part of the C=C double bond in allyl bromide is called a π-bond. Draw a labelled diagram to show the formation of the π-bond. [2] (b)
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education GREEK 0543/04 Paper 4 Writing For Examination from 2015 SPECIMEN PAPER Candidates answer on the Question
Cambridge International Examinations Cambridge International General Certificate of Secondary Education GREEK 0543/04 Paper 4 Writing For Examination from 2015 SPECIMEN PAPER Candidates answer on the Question
Tuesday 4 June 2013 Afternoon
 Tuesday 4 June 2013 Afternoon AS GCE CHEMISTRY A F322/01 Chains, Energy and Resources *F314620613* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A (inserted)
Tuesday 4 June 2013 Afternoon AS GCE CHEMISTRY A F322/01 Chains, Energy and Resources *F314620613* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A (inserted)
Potential Dividers. 46 minutes. 46 marks. Page 1 of 11
 Potential Dividers 46 minutes 46 marks Page 1 of 11 Q1. In the circuit shown in the figure below, the battery, of negligible internal resistance, has an emf of 30 V. The pd across the lamp is 6.0 V and
Potential Dividers 46 minutes 46 marks Page 1 of 11 Q1. In the circuit shown in the figure below, the battery, of negligible internal resistance, has an emf of 30 V. The pd across the lamp is 6.0 V and
Advanced Subsidiary Unit 1: Understanding and Written Response
 Write your name here Surname Other names Edexcel GE entre Number andidate Number Greek dvanced Subsidiary Unit 1: Understanding and Written Response Thursday 16 May 2013 Morning Time: 2 hours 45 minutes
Write your name here Surname Other names Edexcel GE entre Number andidate Number Greek dvanced Subsidiary Unit 1: Understanding and Written Response Thursday 16 May 2013 Morning Time: 2 hours 45 minutes
F322. CHEMISTRY A Chains, Energy and Resources ADVANCED SUBSIDIARY GCE. Friday 27 May 2011 Afternoon. Duration: 1 hour 45 minutes
 ADVANED SUBSIDIARY GE EMISTRY A hains, Energy and Resources F322 *F318530611* andidates answer on the question paper. OR Supplied Materials: Data Sheet for hemistry A (inserted) Other Materials Required:
ADVANED SUBSIDIARY GE EMISTRY A hains, Energy and Resources F322 *F318530611* andidates answer on the question paper. OR Supplied Materials: Data Sheet for hemistry A (inserted) Other Materials Required:
Tuesday 3 June 2014 Afternoon
 Tuesday 3 June 2014 Afternoon AS GCE CHEMISTRY A F322/01 Chains, Energy and Resources *1393437837* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A (inserted)
Tuesday 3 June 2014 Afternoon AS GCE CHEMISTRY A F322/01 Chains, Energy and Resources *1393437837* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A (inserted)
CH 3 CH 2 COOCH 2 CH 2 CH 3 + H 2 O
 Q1.An experiment was carried out to determine the equilibrium constant, K c, for the following reaction. CH 3 CH 2 COOH + CH 3 CH 2 CH 2 OH CH 3 CH 2 COOCH 2 CH 2 CH 3 + H 2 O A student added measured
Q1.An experiment was carried out to determine the equilibrium constant, K c, for the following reaction. CH 3 CH 2 COOH + CH 3 CH 2 CH 2 OH CH 3 CH 2 COOCH 2 CH 2 CH 3 + H 2 O A student added measured
Figure 1 T / K Explain, in terms of molecules, why the first part of the graph in Figure 1 is a line that slopes up from the origin.
 Q1.(a) Figure 1 shows how the entropy of a molecular substance X varies with temperature. Figure 1 T / K (i) Explain, in terms of molecules, why the entropy is zero when the temperature is zero Kelvin.
Q1.(a) Figure 1 shows how the entropy of a molecular substance X varies with temperature. Figure 1 T / K (i) Explain, in terms of molecules, why the entropy is zero when the temperature is zero Kelvin.
Nuclear Physics 5. Name: Date: 8 (1)
 Name: Date: Nuclear Physics 5. A sample of radioactive carbon-4 decays into a stable isotope of nitrogen. As the carbon-4 decays, the rate at which the amount of nitrogen is produced A. decreases linearly
Name: Date: Nuclear Physics 5. A sample of radioactive carbon-4 decays into a stable isotope of nitrogen. As the carbon-4 decays, the rate at which the amount of nitrogen is produced A. decreases linearly
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *9458676952* GREEK 0543/03 Paper 3 Speaking Role Play Card One 1 March 30 April 2015 No Additional
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *9458676952* GREEK 0543/03 Paper 3 Speaking Role Play Card One 1 March 30 April 2015 No Additional
Tuesday 24 January 2012 Afternoon
 Tuesday 24 January 2012 Afternoon A2 GCE PHYSICS A G484 The Newtonian World *G411590112* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships Booklet (sent
Tuesday 24 January 2012 Afternoon A2 GCE PHYSICS A G484 The Newtonian World *G411590112* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships Booklet (sent
(1) Describe the process by which mercury atoms become excited in a fluorescent tube (3)
 Q1. (a) A fluorescent tube is filled with mercury vapour at low pressure. In order to emit electromagnetic radiation the mercury atoms must first be excited. (i) What is meant by an excited atom? (1) (ii)
Q1. (a) A fluorescent tube is filled with mercury vapour at low pressure. In order to emit electromagnetic radiation the mercury atoms must first be excited. (i) What is meant by an excited atom? (1) (ii)
Enthalpy data for the reacting species are given in the table below. The activation energy decreases when the temperature is increased.
 Q1.This question is about the reaction given below. O(g) + H 2O(g) O 2(g) + H 2(g) Enthalpy data for the reacting species are given in the table below. Substance O(g) H 2O(g) O 2(g) H 2(g) ΔH / kj mol
Q1.This question is about the reaction given below. O(g) + H 2O(g) O 2(g) + H 2(g) Enthalpy data for the reacting species are given in the table below. Substance O(g) H 2O(g) O 2(g) H 2(g) ΔH / kj mol
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *6301456813* GREEK 0543/03 Paper 3 Speaking Role Play Card One 1 March 30
www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *6301456813* GREEK 0543/03 Paper 3 Speaking Role Play Card One 1 March 30
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education GREEK 0543/03 Paper 3 Speaking and Listening Role Play Booklet One For Examination from 2011
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education GREEK 0543/03 Paper 3 Speaking and Listening Role Play Booklet One For Examination from 2011
Paper Reference. Paper Reference(s) 6665/01 Edexcel GCE Core Mathematics C3 Advanced. Thursday 11 June 2009 Morning Time: 1 hour 30 minutes
 Centre No. Candidate No. Paper Reference(s) 6665/01 Edexcel GCE Core Mathematics C3 Advanced Thursday 11 June 2009 Morning Time: 1 hour 30 minutes Materials required for examination Mathematical Formulae
Centre No. Candidate No. Paper Reference(s) 6665/01 Edexcel GCE Core Mathematics C3 Advanced Thursday 11 June 2009 Morning Time: 1 hour 30 minutes Materials required for examination Mathematical Formulae
F324. CHEMISTRY A Rings, Polymers and Analysis ADVANCED GCE. Friday 24 June 2011 Morning. Duration: 1 hour
 ADVANCED GCE CHEMISTRY A Rings, Polymers and Analysis F324 *F318640611* Candidates answer on the question paper. OCR supplied materials: Data Sheet for Chemistry A (inserted) Other materials required:
ADVANCED GCE CHEMISTRY A Rings, Polymers and Analysis F324 *F318640611* Candidates answer on the question paper. OCR supplied materials: Data Sheet for Chemistry A (inserted) Other materials required:
the total number of electrons passing through the lamp.
 1. A 12 V 36 W lamp is lit to normal brightness using a 12 V car battery of negligible internal resistance. The lamp is switched on for one hour (3600 s). For the time of 1 hour, calculate (i) the energy
1. A 12 V 36 W lamp is lit to normal brightness using a 12 V car battery of negligible internal resistance. The lamp is switched on for one hour (3600 s). For the time of 1 hour, calculate (i) the energy
Paper Reference. Paper Reference(s) 1776/04 Edexcel GCSE Modern Greek Paper 4 Writing. Thursday 21 May 2009 Afternoon Time: 1 hour 15 minutes
 Centre No. Candidate No. Paper Reference(s) 1776/04 Edexcel GCSE Modern Greek Paper 4 Writing Thursday 21 May 2009 Afternoon Time: 1 hour 15 minutes Materials required for examination Nil Paper Reference
Centre No. Candidate No. Paper Reference(s) 1776/04 Edexcel GCSE Modern Greek Paper 4 Writing Thursday 21 May 2009 Afternoon Time: 1 hour 15 minutes Materials required for examination Nil Paper Reference
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level * 6 3 1 7 7 7 6 4 0 6 * MATHEMATICS (SYLLABUS D) 4024/21 Paper 2 October/November 2013 Candidates answer
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level * 6 3 1 7 7 7 6 4 0 6 * MATHEMATICS (SYLLABUS D) 4024/21 Paper 2 October/November 2013 Candidates answer
Modern Greek Extension
 Centre Number 2017 HIGHER SCHOOL CERTIFICATE EXAMINATION Student Number Modern Greek Extension Written Examination General Instructions Reading time 10 minutes Working time 1 hour and 50 minutes Write
Centre Number 2017 HIGHER SCHOOL CERTIFICATE EXAMINATION Student Number Modern Greek Extension Written Examination General Instructions Reading time 10 minutes Working time 1 hour and 50 minutes Write
The table shows some standard enthalpy of formation data.
 Q1.Vanadium is an important metal. Ferrovanadium, an alloy of iron and vanadium, is used to make a strong type of vanadium-steel. Pure vanadium is used in nuclear reactors. (a) The table shows some standard
Q1.Vanadium is an important metal. Ferrovanadium, an alloy of iron and vanadium, is used to make a strong type of vanadium-steel. Pure vanadium is used in nuclear reactors. (a) The table shows some standard
Wednesday 20 May 2015 Afternoon
 Oxford Cambridge and RSA Wednesday 20 May 2015 Afternoon LEVEL 3 CERTIFICATE ENGINEERING H865/01 Mathematical Techniques and Applications for Engineers * 5 1 0 8 5 0 0 8 2 4 * Candidates answer on the
Oxford Cambridge and RSA Wednesday 20 May 2015 Afternoon LEVEL 3 CERTIFICATE ENGINEERING H865/01 Mathematical Techniques and Applications for Engineers * 5 1 0 8 5 0 0 8 2 4 * Candidates answer on the
THIS IS A NEW SPECIFICATION
 THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE CHEMISTRY A Chains, Energy and Resources F322 * OCE / 1 9 2 3 4* Candidates answer on the Question Paper OCR Supplied Materials: Data Sheet for Chemistry
THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE CHEMISTRY A Chains, Energy and Resources F322 * OCE / 1 9 2 3 4* Candidates answer on the Question Paper OCR Supplied Materials: Data Sheet for Chemistry
Wednesday 19 June 2013 Morning
 Wednesday 19 June 2013 Morning A2 GE EMISTRY A F324/01 Rings, Polymers and Analysis *F314730613* andidates answer on the Question Paper. R supplied materials: Data Sheet for hemistry A (inserted) ther
Wednesday 19 June 2013 Morning A2 GE EMISTRY A F324/01 Rings, Polymers and Analysis *F314730613* andidates answer on the Question Paper. R supplied materials: Data Sheet for hemistry A (inserted) ther
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level * 0 5 1 0 3 4 8 7 8 5 * MATHEMATICS (SYLLABUS D) 4024/21 Paper 2 May/June 2013 Candidates answer on the
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certificate of Education Ordinary Level * 0 5 1 0 3 4 8 7 8 5 * MATHEMATICS (SYLLABUS D) 4024/21 Paper 2 May/June 2013 Candidates answer on the
Thursday 12 January 2012 Afternoon
 Thursday 12 January 2012 Afternoon AS GCE PHYSICS A G481 Mechanics *G411560112* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships Booklet (sent with general
Thursday 12 January 2012 Afternoon AS GCE PHYSICS A G481 Mechanics *G411560112* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships Booklet (sent with general
F321. CHEMISTRY A Atoms, Bonds and Groups ADVANCED SUBSIDIARY GCE. Thursday 13 January 2011 Morning. Duration: 1 hour
 ADVANCED SUBSIDIARY GCE CHEMISTRY A Atoms, Bonds and Groups F321 * OCE / 2 5757* Candidates answer on the question paper. OCR supplied materials: Data Sheet for Chemistry A (inserted) Other materials required:
ADVANCED SUBSIDIARY GCE CHEMISTRY A Atoms, Bonds and Groups F321 * OCE / 2 5757* Candidates answer on the question paper. OCR supplied materials: Data Sheet for Chemistry A (inserted) Other materials required:
CH3HP. (Jun13CH3HP01) General Certificate of Secondary Education Higher Tier June 2013. Unit Chemistry C3. Written Paper TOTAL. Time allowed 1 hour
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Chemistry General Certificate of Secondary Education igher Tier June 2013 C3P Question 1 2
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Chemistry General Certificate of Secondary Education igher Tier June 2013 C3P Question 1 2
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education GREEK 0543/03 Paper 3 Speaking Role Play Card One 1 March 30 April 2010
www.xtremepapers.com UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education GREEK 0543/03 Paper 3 Speaking Role Play Card One 1 March 30 April 2010
THIS IS A NEW SPECIFICATION
 THIS IS A NEW SPECIFICATIN ADVANCED GCE CHEMISTRY A Rings, Polymers and Analysis F324 * CE / 1 5373* Candidates answer on the Question Paper CR Supplied Materials: Data Sheet for Chemistry A (inserted)
THIS IS A NEW SPECIFICATIN ADVANCED GCE CHEMISTRY A Rings, Polymers and Analysis F324 * CE / 1 5373* Candidates answer on the Question Paper CR Supplied Materials: Data Sheet for Chemistry A (inserted)
ΚΥΠΡΙΑΚΗ ΕΤΑΙΡΕΙΑ ΠΛΗΡΟΦΟΡΙΚΗΣ CYPRUS COMPUTER SOCIETY ΠΑΓΚΥΠΡΙΟΣ ΜΑΘΗΤΙΚΟΣ ΔΙΑΓΩΝΙΣΜΟΣ ΠΛΗΡΟΦΟΡΙΚΗΣ 19/5/2007
 Οδηγίες: Να απαντηθούν όλες οι ερωτήσεις. Αν κάπου κάνετε κάποιες υποθέσεις να αναφερθούν στη σχετική ερώτηση. Όλα τα αρχεία που αναφέρονται στα προβλήματα βρίσκονται στον ίδιο φάκελο με το εκτελέσιμο
Οδηγίες: Να απαντηθούν όλες οι ερωτήσεις. Αν κάπου κάνετε κάποιες υποθέσεις να αναφερθούν στη σχετική ερώτηση. Όλα τα αρχεία που αναφέρονται στα προβλήματα βρίσκονται στον ίδιο φάκελο με το εκτελέσιμο
THIS IS A NEW SPECIFICATION
 TIS IS A EW SPEIFIATI ADVAED GE EMISTRY A Rings, Polymers and Analysis F324 * E / 1 5371* andidates answer on the Question Paper R Supplied Materials: Data Sheet for hemistry A (inserted) ther Materials
TIS IS A EW SPEIFIATI ADVAED GE EMISTRY A Rings, Polymers and Analysis F324 * E / 1 5371* andidates answer on the Question Paper R Supplied Materials: Data Sheet for hemistry A (inserted) ther Materials
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education GREEK 0543/03 Paper 3 Speaking Role Play Card One For Examination from 2015 SPECIMEN ROLE PLAY Approx.
Cambridge International Examinations Cambridge International General Certificate of Secondary Education GREEK 0543/03 Paper 3 Speaking Role Play Card One For Examination from 2015 SPECIMEN ROLE PLAY Approx.
PHYA1. General Certificate of Education Advanced Subsidiary Examination June 2012. Particles, Quantum Phenomena and Electricity
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Physics A Unit 1 For this paper you must have: l a pencil and a ruler l a calculator l a Data
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Physics A Unit 1 For this paper you must have: l a pencil and a ruler l a calculator l a Data
Friday 25 May 2012 Afternoon
 Friday 25 May 2012 Afternoon AS GCE PHYSICS A G482 Electrons, Waves and Photons *G411720612* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships Booklet (sent
Friday 25 May 2012 Afternoon AS GCE PHYSICS A G482 Electrons, Waves and Photons *G411720612* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships Booklet (sent
[1] P Q. Fig. 3.1
![[1] P Q. Fig. 3.1 [1] P Q. Fig. 3.1](/thumbs/79/80362156.jpg) 1 (a) Define resistance....... [1] (b) The smallest conductor within a computer processing chip can be represented as a rectangular block that is one atom high, four atoms wide and twenty atoms long. One
1 (a) Define resistance....... [1] (b) The smallest conductor within a computer processing chip can be represented as a rectangular block that is one atom high, four atoms wide and twenty atoms long. One
Math 6 SL Probability Distributions Practice Test Mark Scheme
 Math 6 SL Probability Distributions Practice Test Mark Scheme. (a) Note: Award A for vertical line to right of mean, A for shading to right of their vertical line. AA N (b) evidence of recognizing symmetry
Math 6 SL Probability Distributions Practice Test Mark Scheme. (a) Note: Award A for vertical line to right of mean, A for shading to right of their vertical line. AA N (b) evidence of recognizing symmetry
INSTRUCTIONS TO CANDIDATES
 SPECIMEN EL Entry Level Certificate in Classical Greek R446: Language Test 1: Vocabulary, Grammar and Origins of Words Candidates answer on the Question Paper OCR Supplied Materials: None Duration: 20
SPECIMEN EL Entry Level Certificate in Classical Greek R446: Language Test 1: Vocabulary, Grammar and Origins of Words Candidates answer on the Question Paper OCR Supplied Materials: None Duration: 20
Paper Reference. Paper Reference(s) 1776/01 Edexcel GCSE Modern Greek Paper 1 Listening and Responding
 Centre No. Candidate No. Paper Reference 1 7 7 6 0 1 Surname Signature Paper Reference(s) 1776/01 Edexcel GCSE Modern Greek Paper 1 Listening and Responding Thursday 24 May 2007 Morning Time: 45 minutes
Centre No. Candidate No. Paper Reference 1 7 7 6 0 1 Surname Signature Paper Reference(s) 1776/01 Edexcel GCSE Modern Greek Paper 1 Listening and Responding Thursday 24 May 2007 Morning Time: 45 minutes
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 www.xtremepapers.com UNIVERSITY OF CMRIDGE INTERNTIONL EXMINTIONS International General Certificate of Secondary Education *3788429633* GREEK 0543/03 Paper 3 Speaking and Listening Role Play ooklet One
www.xtremepapers.com UNIVERSITY OF CMRIDGE INTERNTIONL EXMINTIONS International General Certificate of Secondary Education *3788429633* GREEK 0543/03 Paper 3 Speaking and Listening Role Play ooklet One
Thursday 15 June 2017 Morning
 Oxford Cambridge and RSA Thursday 15 June 2017 Morning GCSE CLASSICAL GREEK B402/01 Classical Greek Language 2 (History) *6746000396* Candidates answer on the Question Paper. OCR supplied materials: None
Oxford Cambridge and RSA Thursday 15 June 2017 Morning GCSE CLASSICAL GREEK B402/01 Classical Greek Language 2 (History) *6746000396* Candidates answer on the Question Paper. OCR supplied materials: None
Thursday 17 May 2012 Morning
 Thursday 17 May 2012 Morning AS GCE PHYSICS A G481 Mechanics *G411700612* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships Booklet (sent with general stationery)
Thursday 17 May 2012 Morning AS GCE PHYSICS A G481 Mechanics *G411700612* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships Booklet (sent with general stationery)
F324. CHEMISTRY A Rings, Polymers and Analysis ADVANCED GCE. Wednesday 26 January 2011 Morning. Duration: 1 hour
 ADVANCED GCE CHEMISTRY A Rings, Polymers and Analysis F324 *CE/25761* Candidates answer on the question paper. CR supplied materials: Data Sheet for Chemistry A (inserted) ther materials required: Scientific
ADVANCED GCE CHEMISTRY A Rings, Polymers and Analysis F324 *CE/25761* Candidates answer on the question paper. CR supplied materials: Data Sheet for Chemistry A (inserted) ther materials required: Scientific
Paper Reference. Paper Reference(s) 1776/01 Edexcel GCSE Modern Greek Paper 1 Listening and Responding
 Centre No. Candidate No. Paper Reference 1 7 7 6 0 1 Surname Signature Paper Reference(s) 1776/01 Edexcel GCSE Modern Greek Paper 1 Listening and Responding Friday 18 June 2010 Morning Time: 45 minutes
Centre No. Candidate No. Paper Reference 1 7 7 6 0 1 Surname Signature Paper Reference(s) 1776/01 Edexcel GCSE Modern Greek Paper 1 Listening and Responding Friday 18 June 2010 Morning Time: 45 minutes
Monday 14 January 2013 Afternoon
 Monday 14 January 2013 Afternoon A2 GE EMISTRY A F324/01 Rings, Polymers and Analysis *F314460113* andidates answer on the Question Paper. R supplied materials: Data Sheet for hemistry A (inserted) ther
Monday 14 January 2013 Afternoon A2 GE EMISTRY A F324/01 Rings, Polymers and Analysis *F314460113* andidates answer on the Question Paper. R supplied materials: Data Sheet for hemistry A (inserted) ther
Wednesday 12 June 2013 Afternoon
 Wednesday 12 June 2013 Afternoon A2 GCE CHEMISTRY A F325/01 Equilibria, Energetics and Elements *F314750613* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A
Wednesday 12 June 2013 Afternoon A2 GCE CHEMISTRY A F325/01 Equilibria, Energetics and Elements *F314750613* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A
Paper Reference R. Statistics S1 Advanced/Advanced Subsidiary. Tuesday 10 June 2014 Morning Time: 1 hour 30 minutes
 Centre No. Candidate No. Paper Reference(s) 6683/01R Edexcel GCE Statistics S1 Advanced/Advanced Subsidiary Tuesday 10 June 2014 Morning Time: 1 hour 30 minutes Materials required for examination Mathematical
Centre No. Candidate No. Paper Reference(s) 6683/01R Edexcel GCE Statistics S1 Advanced/Advanced Subsidiary Tuesday 10 June 2014 Morning Time: 1 hour 30 minutes Materials required for examination Mathematical
PHYA1 (JAN12PHYA101) General Certificate of Education Advanced Subsidiary Examination January 2012. Particles, Quantum Phenomena and Electricity TOTAL
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Physics A Unit 1 For this paper you must have: l a pencil and a ruler l a calculator l a Data
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Physics A Unit 1 For this paper you must have: l a pencil and a ruler l a calculator l a Data
physicsandmathstutor.com Paper Reference Core Mathematics C4 Advanced Level Tuesday 23 January 2007 Afternoon Time: 1 hour 30 minutes
 Centre No. Candidate No. Paper Reference(s) 6666/01 Edexcel GCE Core Mathematics C4 Advanced Level Tuesday 23 January 2007 Afternoon Time: 1 hour 30 minutes Materials required for examination Mathematical
Centre No. Candidate No. Paper Reference(s) 6666/01 Edexcel GCE Core Mathematics C4 Advanced Level Tuesday 23 January 2007 Afternoon Time: 1 hour 30 minutes Materials required for examination Mathematical
CHAPTER 25 SOLVING EQUATIONS BY ITERATIVE METHODS
 CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
F211. BIOLOGY Cells, Exchange and Transport ADVANCED SUBSIDIARY GCE. Tuesday 11 January 2011 Morning
 ADVANCED SUBSIDIARY GCE BIOLOGY Cells, Exchange and Transport F211 *OCE/25767* Candidates answer on the question paper. OCR supplied materials: Insert (inserted) Other materials required: Electronic calculator
ADVANCED SUBSIDIARY GCE BIOLOGY Cells, Exchange and Transport F211 *OCE/25767* Candidates answer on the question paper. OCR supplied materials: Insert (inserted) Other materials required: Electronic calculator
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education GREEK 0543/03 Paper 3 Speaking Role Play Card One 1 March 30 April 2009 *8775605210* No Additional
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education GREEK 0543/03 Paper 3 Speaking Role Play Card One 1 March 30 April 2009 *8775605210* No Additional
Phys460.nb Solution for the t-dependent Schrodinger s equation How did we find the solution? (not required)
 Phys460.nb 81 ψ n (t) is still the (same) eigenstate of H But for tdependent H. The answer is NO. 5.5.5. Solution for the tdependent Schrodinger s equation If we assume that at time t 0, the electron starts
Phys460.nb 81 ψ n (t) is still the (same) eigenstate of H But for tdependent H. The answer is NO. 5.5.5. Solution for the tdependent Schrodinger s equation If we assume that at time t 0, the electron starts
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *5654976718* ISLAMIYAT 0493/12 Paper 1 May/June 2015 1 hour 30 minutes Candidates answer on the Question
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *5654976718* ISLAMIYAT 0493/12 Paper 1 May/June 2015 1 hour 30 minutes Candidates answer on the Question
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CMRIDGE INTERNTIONL EXMINTIONS International General Certificate of Secondary Education *3788429633* GREEK 0543/03 Paper 3 Speaking and Listening Role Play ooklet One 1 March 30 pril 2012
UNIVERSITY OF CMRIDGE INTERNTIONL EXMINTIONS International General Certificate of Secondary Education *3788429633* GREEK 0543/03 Paper 3 Speaking and Listening Role Play ooklet One 1 March 30 pril 2012
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *3282737091* GREEK 0543/03 Paper 3 Speaking Role Play Card One 1 March - 30 April 2008 No Additional
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *3282737091* GREEK 0543/03 Paper 3 Speaking Role Play Card One 1 March - 30 April 2008 No Additional
INSTRUCTIONS TO CANDIDATES
 SPECIMEN EL Entry Level Certificate in Classical Greek R446: Language Test 2: Comprehension and Translation Skills Candidates answer on the Question Paper OCR Supplied Materials: None Duration: 20 minutes
SPECIMEN EL Entry Level Certificate in Classical Greek R446: Language Test 2: Comprehension and Translation Skills Candidates answer on the Question Paper OCR Supplied Materials: None Duration: 20 minutes
THIS IS A NEW SPECIFICATION
 THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE PHYSICS A Mechanics G481 *OCE/T69962* Candidates answer on the question paper OCR Supplied Materials: Formulae, Data and Relationships Booklet Other
THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE PHYSICS A Mechanics G481 *OCE/T69962* Candidates answer on the question paper OCR Supplied Materials: Formulae, Data and Relationships Booklet Other
Paper Reference. Modern Greek Paper 1 Listening and Responding. Friday 15 May 2009 Afternoon Time: 45 minutes (+5 minutes reading time)
 Centre No. Candidate No. Paper Reference 1 7 7 6 0 1 Surname Signature Paper Reference(s) 1776/01 Edexcel GCSE Modern Greek Paper 1 Listening and Responding Friday 15 May 2009 Afternoon Time: 45 minutes
Centre No. Candidate No. Paper Reference 1 7 7 6 0 1 Surname Signature Paper Reference(s) 1776/01 Edexcel GCSE Modern Greek Paper 1 Listening and Responding Friday 15 May 2009 Afternoon Time: 45 minutes
derivation of the Laplacian from rectangular to spherical coordinates
 derivation of the Laplacian from rectangular to spherical coordinates swapnizzle 03-03- :5:43 We begin by recognizing the familiar conversion from rectangular to spherical coordinates (note that φ is used
derivation of the Laplacian from rectangular to spherical coordinates swapnizzle 03-03- :5:43 We begin by recognizing the familiar conversion from rectangular to spherical coordinates (note that φ is used
HOMEWORK 4 = G. In order to plot the stress versus the stretch we define a normalized stretch:
 HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
CHEM5 (JUN15CHEM501) General Certificate of Education Advanced Level Examination June 2015. Energetics, Redox and Inorganic Chemistry
 Centre Number Candidate Number For Examiner s Use Surname Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination June 2015 Question 1 2 Mark Chemistry
Centre Number Candidate Number For Examiner s Use Surname Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination June 2015 Question 1 2 Mark Chemistry
Volume of a Cuboid. Volume = length x breadth x height. V = l x b x h. The formula for the volume of a cuboid is
 Volume of a Cuboid The formula for the volume of a cuboid is Volume = length x breadth x height V = l x b x h Example Work out the volume of this cuboid 10 cm 15 cm V = l x b x h V = 15 x 6 x 10 V = 900cm³
Volume of a Cuboid The formula for the volume of a cuboid is Volume = length x breadth x height V = l x b x h Example Work out the volume of this cuboid 10 cm 15 cm V = l x b x h V = 15 x 6 x 10 V = 900cm³
Friday 20 May 2016 Afternoon
 Oxford Cambridge and RSA Friday 20 May 2016 Afternoon GCSE PERSIAN A823/01 Reading *4988529402* Candidates answer on the Question Paper. OCR supplied materials: None Other materials required: None Duration:
Oxford Cambridge and RSA Friday 20 May 2016 Afternoon GCSE PERSIAN A823/01 Reading *4988529402* Candidates answer on the Question Paper. OCR supplied materials: None Other materials required: None Duration:
Unit 4 Further Physical and Organic Chemistry
 Surname Other Names Leave blank Centre Number Candidate Number Candidate Signature General Certificate of Education January 2002 Advanced Level Examination CHEMISTRY Unit 4 Further Physical and Organic
Surname Other Names Leave blank Centre Number Candidate Number Candidate Signature General Certificate of Education January 2002 Advanced Level Examination CHEMISTRY Unit 4 Further Physical and Organic
DESIGN OF MACHINERY SOLUTION MANUAL h in h 4 0.
 DESIGN OF MACHINERY SOLUTION MANUAL -7-1! PROBLEM -7 Statement: Design a double-dwell cam to move a follower from to 25 6, dwell for 12, fall 25 and dwell for the remader The total cycle must take 4 sec
DESIGN OF MACHINERY SOLUTION MANUAL -7-1! PROBLEM -7 Statement: Design a double-dwell cam to move a follower from to 25 6, dwell for 12, fall 25 and dwell for the remader The total cycle must take 4 sec
Instruction Execution Times
 1 C Execution Times InThisAppendix... Introduction DL330 Execution Times DL330P Execution Times DL340 Execution Times C-2 Execution Times Introduction Data Registers This appendix contains several tables
1 C Execution Times InThisAppendix... Introduction DL330 Execution Times DL330P Execution Times DL340 Execution Times C-2 Execution Times Introduction Data Registers This appendix contains several tables
Approximation of distance between locations on earth given by latitude and longitude
 Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
Wednesday 13 June 2012 Morning
 Wednesday 13 June 2012 Morning A2 GCE CHEMISTRY A F325 Equilibria, Energetics and Elements *F314750612* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A (inserted)
Wednesday 13 June 2012 Morning A2 GCE CHEMISTRY A F325 Equilibria, Energetics and Elements *F314750612* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A (inserted)
Section 1: Listening and responding. Presenter: Niki Farfara MGTAV VCE Seminar 7 August 2016
 Section 1: Listening and responding Presenter: Niki Farfara MGTAV VCE Seminar 7 August 2016 Section 1: Listening and responding Section 1: Listening and Responding/ Aκουστική εξέταση Στο πρώτο μέρος της
Section 1: Listening and responding Presenter: Niki Farfara MGTAV VCE Seminar 7 August 2016 Section 1: Listening and responding Section 1: Listening and Responding/ Aκουστική εξέταση Στο πρώτο μέρος της
CHEM4. General Certificate of Education Advanced Level Examination January 2010. Unit 4 Kinetics, Equilibria and Organic Chemistry
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2010 Question 1 2 Mark
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2010 Question 1 2 Mark
CHEM5. General Certificate of Education Advanced Level Examination June 2011. Unit 5 Energetics, Redox and Inorganic Chemistry
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination June 2011 Question 1 2 Mark Chemistry
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination June 2011 Question 1 2 Mark Chemistry
EE512: Error Control Coding
 EE512: Error Control Coding Solution for Assignment on Finite Fields February 16, 2007 1. (a) Addition and Multiplication tables for GF (5) and GF (7) are shown in Tables 1 and 2. + 0 1 2 3 4 0 0 1 2 3
EE512: Error Control Coding Solution for Assignment on Finite Fields February 16, 2007 1. (a) Addition and Multiplication tables for GF (5) and GF (7) are shown in Tables 1 and 2. + 0 1 2 3 4 0 0 1 2 3
Homework 3 Solutions
 Homework 3 Solutions Igor Yanovsky (Math 151A TA) Problem 1: Compute the absolute error and relative error in approximations of p by p. (Use calculator!) a) p π, p 22/7; b) p π, p 3.141. Solution: For
Homework 3 Solutions Igor Yanovsky (Math 151A TA) Problem 1: Compute the absolute error and relative error in approximations of p by p. (Use calculator!) a) p π, p 22/7; b) p π, p 3.141. Solution: For
In your answer, you should make clear how evidence for the size of the nucleus follows from your description
 1. Describe briefly one scattering experiment to investigate the size of the nucleus of the atom. Include a description of the properties of the incident radiation which makes it suitable for this experiment.
1. Describe briefly one scattering experiment to investigate the size of the nucleus of the atom. Include a description of the properties of the incident radiation which makes it suitable for this experiment.
Enthalpy and Entropy
 F35 Equilibria, Energetics & Elements Enthalpy and Entropy 1. A Born Haber cycle for the formation of calcium sulphide is shown below. The cycle includes enthalpy changes for all Steps except Step F. (The
F35 Equilibria, Energetics & Elements Enthalpy and Entropy 1. A Born Haber cycle for the formation of calcium sulphide is shown below. The cycle includes enthalpy changes for all Steps except Step F. (The
Living and Nonliving Created by: Maria Okraska
 Living and Nonliving Created by: Maria Okraska http://enchantingclassroom.blogspot.com Living Living things grow, change, and reproduce. They need air, water, food, and a place to live in order to survive.
Living and Nonliving Created by: Maria Okraska http://enchantingclassroom.blogspot.com Living Living things grow, change, and reproduce. They need air, water, food, and a place to live in order to survive.
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 ambridge International Examinations ambridge International General ertificate of Secondary Education *8412306169* GREEK 0543/03 Paper 3 Speaking and Listening Role Play ooklet One 1 March 30 pril 2014
ambridge International Examinations ambridge International General ertificate of Secondary Education *8412306169* GREEK 0543/03 Paper 3 Speaking and Listening Role Play ooklet One 1 March 30 pril 2014
2 Composition. Invertible Mappings
 Arkansas Tech University MATH 4033: Elementary Modern Algebra Dr. Marcel B. Finan Composition. Invertible Mappings In this section we discuss two procedures for creating new mappings from old ones, namely,
Arkansas Tech University MATH 4033: Elementary Modern Algebra Dr. Marcel B. Finan Composition. Invertible Mappings In this section we discuss two procedures for creating new mappings from old ones, namely,
Calculating the propagation delay of coaxial cable
 Your source for quality GNSS Networking Solutions and Design Services! Page 1 of 5 Calculating the propagation delay of coaxial cable The delay of a cable or velocity factor is determined by the dielectric
Your source for quality GNSS Networking Solutions and Design Services! Page 1 of 5 Calculating the propagation delay of coaxial cable The delay of a cable or velocity factor is determined by the dielectric
Tuesday 17 June 2014 Afternoon
 Tuesday 17 June 2014 Afternoon A2 GCE CHEMISTRY A F325/01 Equilibria, Energetics and Elements *3175284854* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A (inserted)
Tuesday 17 June 2014 Afternoon A2 GCE CHEMISTRY A F325/01 Equilibria, Energetics and Elements *3175284854* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A (inserted)
Solutions to the Schrodinger equation atomic orbitals. Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz
 Solutions to the Schrodinger equation atomic orbitals Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz ybridization Valence Bond Approach to bonding sp 3 (Ψ 2 s + Ψ 2 px + Ψ 2 py + Ψ 2 pz) sp 2 (Ψ 2 s + Ψ 2 px + Ψ 2 py)
Solutions to the Schrodinger equation atomic orbitals Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz ybridization Valence Bond Approach to bonding sp 3 (Ψ 2 s + Ψ 2 px + Ψ 2 py + Ψ 2 pz) sp 2 (Ψ 2 s + Ψ 2 px + Ψ 2 py)
Wednesday 11 June 2014 Afternoon
 Wednesday 11 June 2014 Afternoon A2 GCE PHYSICS A G484/01 The Newtonian World *3270355010* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships Booklet (sent
Wednesday 11 June 2014 Afternoon A2 GCE PHYSICS A G484/01 The Newtonian World *3270355010* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships Booklet (sent
PHYA1 (JUN13PHYA101) General Certificate of Education Advanced Subsidiary Examination June 2013. Particles, Quantum Phenomena and Electricity TOTAL
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Physics A Unit 1 For this paper you must have: l a pencil and a ruler l a calculator l a Data
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials Physics A Unit 1 For this paper you must have: l a pencil and a ruler l a calculator l a Data
F214. BIOLOGY Communication, Homeostasis and Energy ADVANCED GCE. Monday 24 January 2011 Afternoon
 ADVANCED GCE BIOLOGY Communication, Homeostasis and Energy F214 * OCE / 2 5763* Candidates answer on the question paper. OCR supplied materials: None Other materials required: Electronic calculator Ruler
ADVANCED GCE BIOLOGY Communication, Homeostasis and Energy F214 * OCE / 2 5763* Candidates answer on the question paper. OCR supplied materials: None Other materials required: Electronic calculator Ruler
Assalamu `alaikum wr. wb.
 LUMP SUM Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. LUMP SUM Lump sum lump sum lump sum. lump sum fixed price lump sum lump
LUMP SUM Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. LUMP SUM Lump sum lump sum lump sum. lump sum fixed price lump sum lump
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 ambridge International Examinations ambridge International General ertificate of Secondary Education *2022097877* GREEK 0543/01 Paper 1 Listening May/June 2015 pprox. 45 minutes andidates answer on the
ambridge International Examinations ambridge International General ertificate of Secondary Education *2022097877* GREEK 0543/01 Paper 1 Listening May/June 2015 pprox. 45 minutes andidates answer on the
ΚΥΠΡΙΑΚΟΣ ΣΥΝΔΕΣΜΟΣ ΠΛΗΡΟΦΟΡΙΚΗΣ CYPRUS COMPUTER SOCIETY 21 ος ΠΑΓΚΥΠΡΙΟΣ ΜΑΘΗΤΙΚΟΣ ΔΙΑΓΩΝΙΣΜΟΣ ΠΛΗΡΟΦΟΡΙΚΗΣ Δεύτερος Γύρος - 30 Μαρτίου 2011
 Διάρκεια Διαγωνισμού: 3 ώρες Απαντήστε όλες τις ερωτήσεις Μέγιστο Βάρος (20 Μονάδες) Δίνεται ένα σύνολο από N σφαιρίδια τα οποία δεν έχουν όλα το ίδιο βάρος μεταξύ τους και ένα κουτί που αντέχει μέχρι
Διάρκεια Διαγωνισμού: 3 ώρες Απαντήστε όλες τις ερωτήσεις Μέγιστο Βάρος (20 Μονάδες) Δίνεται ένα σύνολο από N σφαιρίδια τα οποία δεν έχουν όλα το ίδιο βάρος μεταξύ τους και ένα κουτί που αντέχει μέχρι
6.1. Dirac Equation. Hamiltonian. Dirac Eq.
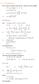 6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
