metal-organic compounds
|
|
|
- Ἀγάπιος Μαυρογένης
- 6 χρόνια πριν
- Προβολές:
Transcript
1 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN {Bis[2-(diphenylphosphanyl)phenyl] ether-j 2 P,P 0 }(1,1 0 -dibenzyl-1h,1 0 H-4,4 0 - bi-1,2,3-triazole-j 2 N 3,N 30 )copper(i) hexafluoridophosphate dichloromethane hemisolvate Uwe Monkowius, a * Stefan Ritter, b Burkhard König, b Hartmut Yersin c and Manfred Zabel d a Johannes Kepler Universität Linz, Institut für Anorganische Chemie, Altenbergerstr. 69, 4040 Linz, Austria, b Universität Regensburg, Institut für Organische Chemie, Universitätsstr. 31, Regensburg, Germany, c Universität Regensburg, Institut für Physikalische Chemie, Universitätsstr. 31, Regensburg, Germany, and d Universität Regensburg, Zentrale Analytik, Röntgenstrukturanalyse, Universitätsstr. 31, Regensburg, Germany Correspondence uwe.monkowius@jku.at Received 26 November 2007; accepted 10 December 2007 Key indicators: single-crystal X-ray study; T = 123 K; mean (C C) = Å; some non-h atoms missing; disorder in solvent or counterion; R factor = 0.045; wr factor = 0.109; data-to-parameter ratio = Experimental Crystal data [Cu(C 18 H 16 N 6 )(C 36 H 28 OP 2 )]PF 6-0.5CH 2 Cl 2 M r = Triclinic, P1 a = (1) Å b = (1) Å c = (3) Å = (1) Data collection Stoe IPDS diffractometer Absorption correction: analytical (X-SHAPE and X-RED in IPDS Software; Stoe & Cie, 1998) T min = 0.846, T max = Refinement R[F 2 >2(F 2 )] = wr(f 2 ) = S = reflections Table 1 Selected bond lengths (Å). Cu1 P (9) Cu1 P (11) Cu1 N (3) Cu1 N (2) = (1) = (1) V = (9) Å 3 Z =4 Mo K radiation = 0.61 mm 1 T = 123 K mm measured reflections independent reflections reflections with I > 2(I) R int = parameters H-atom parameters constrained max = 1.65 e Å 3 min = 0.32 e Å 3 Cu2 P (10) Cu2 N (3) Cu2 N (3) Cu2 P (8) In the crystal structure of the title compound, [Cu(C 18 H 16 N 6 )(C 36 H 28 OP 2 )]PF 6 0.5CH 2 Cl 2 or [Cu(DPE- Phos)(Bn-bta)]PF 6 0.5CH 2 Cl 2 {DPEPhos = bis[(diphenylphosphanyl)phenyl] ether and Bn-bta = 1,1 0 -dibenzyl- 1H,1 0 H-4,4 0 -bi-1,2,3triazole}, the Cu atom is coordinated by two N and two P atoms of the ligands in a strongly distorted tetrahedral environment. There are two crystallographically independent complex cations present, which differ significantly in their geometrical parameters. The solvent molecule is disordered but satisfactory atomic positions could not be determined. Related literature For related literature, see: Monkowius et al. (2007) and references therein; Cosier & Glazer (1986). For the treatment of disordered solvent, see: Spek (2003). Data collection: IPDS Software (Stoe & Cie, 1998); cell refinement: IPDS Software; data reduction: IPDS Software; program(s) used to solve structure: SIR97 (Altomare et al., 1999); program(s) used to refine structure: SHELXL97 (Sheldrick, 1997); molecular graphics: PLATON (Spek, 2003); software used to prepare material for publication: PLATON. Parts of this work were supported by the Bundesministerium für Bildung und Forschung (BMBF). We also acknowledge financial support from the DFG (SPP 1118). SR thanks the Elitenetzwerk Bayern for a graduate fellowship. Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IM2049). References Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst. 32, Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst. 19, Monkowius, U., Ritter, S., König, B., Zabel, M. & Yersin, H. (2007). Eur. J. Inorg. Chem. pp Sheldrick, G. M. (1997). SHELXL97. University of Göttingen, Germany. Spek, A. L. (2003). J. Appl. Cryst. 36, Stoe & Cie (1998). IPDS Software. Version Stoe & Cie GmbH, Darmstadt, Germany. doi: /s # 2008 International Union of Crystallography m195
2 supporting information [ {Bis[2-(diphenylphosphanyl)phenyl] ether-κ 2 P,P }(1,1 -dibenzyl-1h,1 H-4,4 bi-1,2,3-triazole-κ 2 N 3,N 3 )copper(i) hexafluoridophosphate dichloromethane hemisolvate Uwe Monkowius, Stefan Ritter, Burkhard König, Hartmut Yersin and Manfred Zabel S1. Comment The title compound crystallizes in two forms from dichloromethane/diethyl ether: one form contains solvent molecules, the other not. The latter structure was recently published (Monkowius et al., 2007), the former is now reported in this paper. The asymmetric unit of the title compound consists of two complex cations [(DPEPhos)(Bn-bta)Cu] +, two anions [PF 6 ] -, and one molecule CH 2 Cl 2 (Fig. 1). Additional partially occupied and/or disordered solvent molecules were also found, but satisfactory atomic positions could not be determined. These disordered solvent molecules were treated as a diffuse contribution using the SQUEEZE routine in PLATON software package (Spek, 2003). SQUEEZE calculated Å 3 void space per unit cell and 19.8 electrons. Both cations significantly differ in their geometrical parameters: The Cu P bond lengths are (9)/ (11) Å for Cu1 P1/Cu1 P2 and (9)/ (10) Å for Cu2 P3/Cu2 P4; the Cu N bond lengths are (3)/2.102 (3) Å (Cu1 N1/Cu1 N4) and (3)/2.063 (3) Å (Cu2 N7/Cu2 N10). Furthermore, the bis-triazolyl moiety deviates considerably from planarity in the case of the cation containing Cu1 [Cu1: (N1 C38 C39 N4) = (5) ; Cu2: (N7 C92 C93 N10) = -3.9 (5) ]. The bite angles of the Bnbta ligands are (10) and (10) (N1 Cu1 N4 and N7 Cu2 N10, respectively). There are no close contacts between the oxygen atoms of the phosphine ligands and the copper atoms. The complex cations in the crystal structure of the crystal containing no solvent molecules display comparable sturctural characteristics (Monkowius et al., 2007). S2. Experimental The preparation of the title compound was published recently (Monkowius et al., 2007). S3. Refinement The data were collected at 123 K using an Oxford Cryosystems Cooler (Cosier & Glazer, 1986). The structure was solved by direct methods (SIR97) and refined by full-matrix anisotropic least squares (SHELXL97). The H-atoms were calculated geometrically and a riding model with U iso (H) = 1.2 U eq (C) the was used during refinement process. sup-1
3 Figure 1 Excerpt from a cell plott depicting the hydrogen-bond pattern in crystals of the title compound. Hydrogen bonds are indicated by dashed lines. Only the participating H atoms are shown for clarity. Figure 2 ORTEP drawing of the structure of the cation containing Cu1 in crystals of the title compound (H atoms have been omitted for clarity). Displacement ellipsoids for non-h atoms are drawn at the 50% probability level. sup-2
4 Figure 3 ORTEP drawing of the structure of the cation containing Cu2. Displacement ellipsoids for non-h atoms are drawn at the 50% probability level. {Bis[2-(diphenylphosphanyl)phenyl] ether-κ 2 P,P }(1,1 -dibenzyl-1h,1 H-4,4 -bi- 1,2,3-triazole-κ 2 N,N )copper(i) hexafluoridophosphate dichloromethane hemisolvate Crystal data [Cu(C 18 H 16 N 6 )(C 36 H 28 OP 2 )]PF 6 0.5CH 2 Cl 2 M r = Triclinic, P1 Hall symbol: -P 1 a = (1) Å b = (1) Å c = (3) Å α = (1) β = (1) γ = (1) V = (9) Å 3 Z = 4 Data collection Stoe IPDS diffractometer Radiation source: fine-focus sealed tube Graphite monochromator rotation scans F(000) = 2268 Cell parameters were determined by indexing 8000 reflections with I/sigma limit 6.0. D x = Mg m 3 Mo Kα radiation, λ = Å Cell parameters from 8000 reflections θ = µ = 0.61 mm 1 T = 123 K Prism, colourless mm Absorption correction: analytical (X-SHAPE and X-RED; Stoe, 1998) T min = 0.846, T max = measured reflections independent reflections sup-3
5 12619 reflections with I > 2σ(I) R int = θ max = 26.9, θ min = 2.0 Refinement Refinement on F 2 Least-squares matrix: full R[F 2 > 2σ(F 2 )] = wr(f 2 ) = S = reflections 1306 parameters 0 restraints Primary atom site location: structure-invariant direct methods h = k = l = Secondary atom site location: difference Fourier map Hydrogen site location: inferred from neighbouring sites H-atom parameters constrained w = 1/[σ 2 (F o2 ) + (0.0577P) 2 ] where P = (F o 2 + 2F c2 )/3 (Δ/σ) max = Δρ max = 1.65 e Å 3 Δρ min = 0.32 e Å 3 Special details Experimental. Data were collected applying an imaging plate system (Stoe) with the following measurement parameters: Detector distance [mm] 65 Phi movement mode Oscillation Phi incr. [degrees] 0.8 Number of exposures 267 Irradiation / exposure [min] 3.00 For a detailed description of the method see: Sheldrick, G.M., Paulus, E. Vertesy, L. & Hahn, F. (1995) Acta Cryst. B51, Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell e.s.d.'s are taken into account in the estimation of distances, angles and torsion angles Refinement. Refinement of F 2 against ALL reflections. The weighted R-factor wr and goodness of fit S are based on F 2, conventional R-factors R are based on F, with F set to zero for negative F 2. The threshold expression of F 2 > σ(f 2 ) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F 2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å 2 ) x y z U iso */U eq Occ. (<1) Cu (3) (3) (1) (1) P (6) (5) (3) (3) P (7) (5) (3) (3) O (17) (14) (7) (7) N (2) (17) (8) (8) N (2) (18) (9) (9) N (2) (19) (9) (9) N (2) (17) (8) (8) N (2) (17) (9) (8) N (2) (17) (8) (8) C (3) (2) (10) (10) C (3) (2) (11) (10) C (3) (2) (12) (11) C (3) (2) (13) (11) C (3) (2) (12) (11) C (3) (2) (11) (10) C (3) (2) (10) (10) C (3) (2) (11) (10) sup-4
6 C (3) (3) (13) (13) C (3) (3) (13) (14) C (3) (3) (13) (15) C (3) (3) (11) (11) C (3) (2) (10) (9) C (3) (2) (10) (9) C (3) (2) (11) (10) C (3) (2) (12) (11) C (3) (2) (12) (10) C (3) (2) (11) (10) C (2) (2) (10) (9) C (3) (2) (11) (10) C (3) (2) (11) (10) C (3) (2) (11) (10) C (3) (2) (10) (10) C (2) (19) (10) (9) C (3) (2) (10) (10) C (3) (3) (11) (11) C (3) (3) (12) (15) C (4) (3) (13) (15) C (3) (3) (13) (13) C (3) (2) (12) (11) C (3) (2) (10) (10) C (3) (2) (11) (10) C (3) (2) (12) (11) C (3) (2) (11) (13) C (3) (2) (13) (13) C (3) (2) (12) (11) C (3) (2) (11) (10) C (3) (2) (10) (9) C (3) (2) (10) (9) C (3) (2) (10) (10) C (3) (3) (13) (13) C (3) (2) (13) (11) C (3) (3) (16) (13) C (3) (3) (18) (16) C (3) (3) (18) (16) C (3) (3) (16) (15) C (3) (2) (14) (11) C (3) (2) (11) (10) C (3) (2) (11) (10) C (3) (2) (12) (10) C (3) (2) (14) (11) C (3) (2) (13) (13) C (3) (2) (13) (13) C (3) (2) (12) (10) Cu (3) (2) (1) (1) P (7) (5) (3) (2) sup-5
7 P (7) (5) (2) (2) O (17) (14) (7) (7) N (2) (18) (9) (8) N (2) (18) (9) (9) N (2) (18) (9) (9) N (2) (17) (9) (8) N (2) (18) (9) (9) N (2) (19) (9) (9) C (3) (2) (10) (9) C (3) (2) (11) (10) C (3) (3) (13) (12) C (3) (2) (12) (13) C (3) (2) (13) (13) C (3) (2) (11) (10) C (3) (2) (10) (10) C (3) (2) (11) (12) C (3) (2) (12) (13) C (3) (3) (11) (13) C (3) (3) (12) (13) C (3) (2) (11) (10) C (3) (2) (10) (9) C (3) (2) (10) (10) C (3) (2) (11) (10) C (3) (2) (11) (11) C (3) (2) (10) (10) C (3) (19) (10) (9) C (2) (2) (10) (9) C (3) (2) (10) (10) C (3) (2) (10) (10) C (3) (2) (10) (10) C (3) (2) (10) (10) C (2) (2) (10) (9) C (3) (2) (10) (9) C (3) (2) (11) (10) C (3) (2) (12) (11) C (3) (2) (12) (11) C (3) (2) (11) (10) C (3) (2) (10) (10) C (3) (2) (10) (9) C (3) (2) (11) (10) C (3) (2) (11) (10) C (3) (2) (12) (10) C (3) (2) (12) (13) C (3) (2) (10) (10) C (3) (2) (11) (10) C (3) (2) (10) (10) C (3) (2) (11) (10) C (3) (2) (11) (10) sup-6
8 C (3) (2) (12) (13) C (3) (2) (11) (13) C (4) (3) (13) (13) C (4) (3) (14) (13) C (4) (3) (14) (14) C (4) (3) (13) (14) C (3) (2) (12) (13) C (3) (3) (12) (11) C (3) (2) (11) (10) C (3) (2) (12) (11) C (3) (3) (13) (13) C (4) (3) (12) (14) C (3) (3) (12) (13) C (3) (2) (12) (11) P (9) (9) (4) (4) F (3) (2) (11) (11) F (2) (18) (8) (10) F (3) (2) (11) (11) F (3) (3) (11) (16) F (3) (2) (11) (14) F (3) (4) (12) (2) P (8) (6) (3) (3) F (2) (16) (7) (9) F (17) (13) (7) (7) F (17) (14) (8) (8) F (18) (14) (7) (7) F (19) (15) (7) (8) F (19) (15) (8) (8) Cl (2) (18) (9) (9) Cl (2) (2) (10) (10) C (8) (6) (3) (3) H * H * H * H * H * H * H * H * H * H * H * H * H * H * H * H * H * sup-7
9 H * H * H * H * H * H * H * H * H * H * H * H * H * H41A * H41B * H * H * H * H * H * H48A * H48B * H * H * H * H * H * H10D * H10E * H * H * H * H * H * H * H * H * H * H * H * H * H * H * H * H * H * H * H * sup-8
10 H * H * H * H * H * H * H * H * H * H * H * H95A * H95B * H * H * H * H * H * H * H * H * H * H * H10A * H10B * Atomic displacement parameters (Å 2 ) U 11 U 22 U 33 U 12 U 13 U 23 Cu (2) (2) (2) (2) (2) (1) P (5) (4) (4) (3) (3) (3) P (5) (4) (4) (3) (3) (3) O (13) (10) (11) (9) (9) (8) N (17) (12) (13) (11) (11) (10) N (19) (13) (14) (12) (12) (11) N (19) (13) (15) (12) (12) (11) N (17) (11) (13) (11) (11) (9) N (17) (12) (13) (11) (11) (10) N (17) (12) (13) (11) (11) (10) C (19) (14) (16) (12) (13) (12) C (2) (15) (17) (14) (14) (13) C (2) (17) (18) (15) (15) (14) C (2) (17) (2) (15) (16) (15) C (2) (17) (19) (15) (15) (14) C (2) (16) (17) (14) (14) (13) C (2) (15) (15) (13) (13) (12) C (2) (16) (17) (14) (14) (13) C (3) (18) (2) (17) (19) (15) sup-9
11 C (3) (2) (2) (2) (2) (17) C (3) (3) (19) (2) (18) (18) C (2) (19) (18) (16) (15) (14) C (2) (14) (14) (13) (12) (11) C (2) (14) (14) (13) (12) (11) C (2) (16) (17) (14) (14) (13) C (2) (16) (2) (14) (16) (14) C (2) (15) (19) (14) (16) (13) C (2) (15) (17) (14) (14) (12) C (19) (13) (14) (12) (12) (11) C (2) (15) (15) (13) (13) (12) C (2) (14) (17) (13) (14) (12) C (2) (14) (17) (13) (13) (12) C (2) (14) (15) (13) (13) (11) C (19) (13) (15) (12) (12) (11) C (2) (16) (15) (14) (13) (12) C (2) (19) (16) (16) (14) (14) C (3) (3) (17) (2) (16) (16) C (3) (3) (19) (2) (18) (17) C (3) (18) (2) (18) (19) (16) C (2) (17) (18) (16) (16) (14) C (2) (15) (15) (14) (13) (12) C (2) (16) (17) (15) (14) (13) C (2) (18) (19) (16) (16) (15) C (3) (16) (17) (16) (15) (13) C (3) (18) (2) (18) (18) (15) C (2) (18) (19) (15) (16) (15) C (2) (16) (17) (14) (14) (13) C (2) (14) (14) (13) (12) (11) C (2) (14) (14) (12) (12) (11) C (2) (15) (15) (13) (13) (12) C (3) (18) (2) (16) (17) (15) C (2) (17) (2) (15) (16) (15) C (2) (17) (3) (15) (2) (18) C (3) (2) (3) (19) (2) (2) C (3) (2) (3) (2) (2) (2) C (3) (18) (3) (17) (2) (19) C (2) (17) (2) (16) (18) (16) C (2) (14) (17) (14) (15) (12) C (2) (14) (17) (13) (14) (12) C (2) (16) (18) (15) (16) (13) C (2) (16) (2) (15) (18) (15) C (3) (15) (2) (14) (19) (14) C (3) (15) (19) (16) (18) (13) C (2) (15) (19) (15) (16) (13) Cu (2) (2) (2) (2) (2) (1) P (5) (3) (4) (3) (3) (3) P (5) (3) (4) (3) (3) (3) sup-10
12 O (14) (10) (10) (9) (9) (8) N (18) (12) (13) (11) (11) (10) N (19) (13) (14) (12) (12) (10) N (2) (12) (14) (12) (13) (10) N (17) (12) (13) (11) (11) (10) N (18) (13) (14) (12) (12) (11) N (18) (13) (15) (12) (12) (11) C (2) (14) (14) (13) (12) (11) C (2) (16) (18) (14) (15) (13) C (2) (2) (2) (17) (16) (16) C (3) (16) (2) (16) (17) (14) C (3) (16) (2) (15) (18) (14) C (2) (15) (18) (14) (14) (13) C (2) (14) (15) (13) (12) (11) C (3) (15) (16) (15) (15) (12) C (3) (17) (18) (18) (18) (14) C (3) (19) (16) (18) (16) (14) C (3) (18) (17) (17) (16) (14) C (2) (15) (16) (15) (15) (12) C (2) (13) (15) (12) (13) (11) C (2) (15) (15) (13) (13) (12) C (2) (17) (16) (15) (14) (13) C (2) (17) (18) (15) (15) (14) C (2) (16) (15) (14) (13) (12) C (2) (13) (14) (12) (12) (10) C (19) (14) (14) (12) (12) (11) C (2) (14) (16) (13) (13) (12) C (2) (17) (15) (14) (13) (13) C (2) (16) (15) (14) (13) (12) C (19) (14) (16) (13) (13) (12) C (19) (14) (14) (12) (12) (11) C (2) (13) (15) (12) (13) (11) C (2) (15) (16) (14) (14) (12) C (2) (16) (2) (16) (16) (14) C (2) (17) (19) (16) (16) (14) C (2) (17) (16) (16) (15) (13) C (2) (15) (16) (14) (13) (12) C (2) (14) (14) (12) (12) (11) C (2) (15) (16) (14) (14) (12) C (2) (17) (17) (15) (14) (14) C (2) (15) (18) (14) (15) (13) C (3) (15) (18) (15) (16) (13) C (2) (15) (15) (14) (13) (12) C (2) (15) (18) (14) (15) (13) C (2) (14) (16) (13) (13) (12) C (2) (15) (16) (13) (13) (12) C (2) (16) (18) (14) (14) (13) C (3) (17) (18) (17) (17) (14) sup-11
13 C (3) (16) (16) (16) (16) (13) C (3) (18) (2) (17) (18) (15) C (3) (17) (2) (19) (2) (16) C (3) (2) (2) (2) (2) (17) C (3) (2) (2) (19) (18) (17) C (3) (17) (18) (18) (17) (14) C (2) (18) (19) (16) (16) (15) C (2) (16) (16) (14) (14) (13) C (2) (17) (18) (15) (15) (14) C (3) (17) (2) (16) (17) (15) C (3) (2) (19) (2) (18) (16) C (3) (2) (18) (2) (17) (17) C (2) (17) (18) (15) (16) (14) P (7) (7) (5) (5) (5) (5) F (2) (18) (2) (15) (18) (15) F (2) (15) (15) (13) (13) (12) F (2) (16) (2) (15) (17) (14) F (3) (3) (2) (3) (2) (2) F (3) (2) (2) (18) (2) (18) F (4) (4) (2) (3) (2) (3) P (6) (4) (4) (4) (4) (3) F (2) (12) (12) (12) (11) (10) F (15) (10) (12) (9) (10) (9) F (15) (11) (13) (9) (11) (9) F (16) (10) (11) (10) (10) (8) F (17) (11) (11) (11) (10) (9) F (16) (12) (15) (10) (12) (11) Cl (18) (14) (15) (12) (12) (12) Cl (17) (19) (18) (14) (13) (15) C (7) (4) (5) (5) (5) (4) Geometric parameters (Å, º) Cu1 P (9) C22 H Cu1 P (11) C23 H Cu1 N (3) C26 H Cu1 N (2) C27 H Cu2 P (10) C28 H Cu2 N (3) C29 H Cu2 N (3) C30 H Cu2 P (8) C32 H Cl1 C (9) C33 H Cl2 C (11) C34 H P1 C (3) C35 H P1 C (4) C36 H P1 C (4) C37 H P2 C (3) C40 H P2 C (3) C41 H41A sup-12
14 P2 C (3) C41 H41B P3 C (4) C43 H P3 C (3) C44 H P3 C (3) C45 H P4 C (3) C46 H P4 C (3) C47 H P4 C (4) C48 H48A P5 F (3) C48 H48B P5 F (4) C50 H P5 F (4) C51 H P5 F (4) C52 H P5 F (4) C53 H P5 F (3) C54 H P6 F (2) C55 C (5) P6 F (2) C55 C (6) P6 F (2) C56 C (5) P6 F (3) C57 C (5) P6 F (2) C58 C (6) P6 F (3) C59 C (5) O1 C (3) C61 C (4) O1 C (4) C61 C (4) O2 C (4) C62 C (5) O2 C (3) C63 C (5) N1 N (4) C64 C (6) N1 C (4) C65 C (5) N2 N (4) C67 C (5) N3 C (5) C67 C (5) N3 C (4) C68 C (6) N4 C (4) C69 C (5) N4 N (3) C70 C (5) N5 N (4) C71 C (6) N6 C (4) C73 C (4) N6 C (4) C73 C (4) N7 C (4) C74 C (4) N7 N (4) C75 C (4) N8 N (4) C76 C (4) N9 C (5) C77 C (4) N9 C (4) C79 C (4) N10 N (4) C79 C (5) N10 C (4) C80 C (5) N11 N (4) C81 C (5) N12 C (5) C82 C (5) N12 C (4) C83 C (5) C1 C (4) C85 C (4) C1 C (5) C85 C (5) C2 C (5) C86 C (4) C3 C (5) C87 C (4) C4 C (5) C88 C (5) sup-13
15 C5 C (5) C89 C (4) C7 C (5) C91 C (4) C7 C (4) C92 C (5) C8 C (5) C93 C (5) C9 C (6) C95 C (5) C10 C (6) C96 C (5) C11 C (5) C96 C (6) C13 C (4) C97 C (7) C13 C (6) C98 C (7) C14 C (5) C99 C (6) C15 C (4) C100 C (6) C16 C (6) C102 C (5) C17 C (5) C103 C (5) C19 C (4) C103 C (4) C19 C (5) C104 C (5) C20 C (5) C105 C (6) C21 C (4) C106 C (6) C22 C (5) C107 C (5) C23 C (5) C56 H C25 C (4) C57 H C25 C (5) C58 H C26 C (5) C59 H C27 C (6) C60 H C28 C (6) C62 H C29 C (5) C63 H C31 C (6) C64 H C31 C (5) C65 H C32 C (4) C66 H C33 C (5) C68 H C34 C (6) C69 H C35 C (4) C70 H C37 C (6) C71 H C38 C (4) C74 H C39 C (4) C75 H C41 C (5) C76 H C42 C (6) C77 H C42 C (4) C80 H C43 C (6) C81 H C44 C (6) C82 H C45 C (7) C83 H C46 C (6) C84 H C48 C (5) C86 H C49 C (5) C87 H C49 C (6) C88 H C50 C (6) C89 H C51 C (5) C90 H C52 C (6) C91 H C53 C (6) C94 H sup-14
16 C2 H C95 H95B C3 H C95 H95A C4 H C97 H C5 H C98 H C6 H C99 H C8 H C100 H C9 H C101 H C10 H C102 H10E C11 H C102 H10D C12 H C104 H C15 H C105 H C16 H C106 H C17 H C107 H C18 H C108 H C20 H C109 H10A C21 H C109 H10B Cu1 O (2) C46 H33 ii Cu2 O (2) C46 H Cu1 H C47 H Cu1 H C47 H41B iii Cu1 H C51 H16 ix Cu1 H C52 H Cu2 H C52 H16 ix Cu2 H C53 H Cu2 H C55 H Cl1 H C59 H99 xi P2 C (3) C60 H P3 P (11) C61 H P4 N (3) C62 H98 xi P4 N (3) C62 H P4 C (3) C65 H100 xii P4 P (11) C67 H P6 H94 i C67 H F1 C21 ii (4) C70 H10B viii F2 C21 ii (4) C72 H F2 C (5) C73 H22 ii F2 C47 iii (4) C73 H F3 C (6) C73 H F3 C (4) C74 H22 ii F5 C40 iv (4) C74 H F6 C48 iv (5) C74 H F8 C94 i (5) C75 H22 ii F9 C94 i (4) C75 H F11 C53 iv (5) C76 H22 ii F11 C54 iv (4) C76 H F11 C4 iii (4) C77 H F11 C3 iii (4) C77 H22 ii sup-15
17 F12 C81 iv (4) C78 H F1 H C78 H22 ii F1 H21 ii C79 H F2 H C80 H F2 H21 ii C80 H F2 H47 iii C82 H F3 H C83 H95A xii F3 H C83 H F4 H50 iv C84 H F4 H46 iii C85 H F5 H40 iv C86 H F6 H48A iv C87 H F7 H65 v C88 H17 ix F7 H64 v C88 H F8 H94 i C89 H F8 H54 iv C96 H69 xii F8 H81 iv C97 H69 xii F9 H94 i C98 H F9 H104 i C99 H62 xiii F10 H64 v C100 H F10 H3 iii C101 H83 xii F11 H3 iii C101 H F11 H53 iv C103 H F11 H54 iv C104 H F11 H4 iii C104 H48B ii F12 H81 iv C105 H O1 Cu (2) C106 H28 iv O1 C (5) C106 H O1 C (4) C107 H O2 Cu (2) C108 H O2 C (4) H2 C O2 C (4) H2 C N1 C (4) H3 F10 iii N1 N (3) H3 F11 iii N2 C (4) H4 F11 iii N3 C33 vi (4) H5 C N4 N (3) H5 C N4 C (4) H5 C N7 N (4) H5 C N7 C (5) H6 Cu N7 P (3) H6 N N8 C (4) H6 N N8 C (5) H8 Cu N10 C (4) H8 C N10 N (4) H8 N N10 P (3) H8 C N10 C (4) H9 C N11 C23 ii (4) H9 H sup-16
18 N1 H H10A F9 i N2 H26 vi H10A F10 i N2 H H10D C9 ii N3 H H10D C8 ii N4 H H10D H N4 H H10E H N5 H H12 C N7 H H12 C N8 H H12 H57 vii N8 H H15 C N8 H H15 H44 vii N11 H23 ii H15 C44 vii C2 C (5) H15 C C3 F11 iii (4) H16 C51 viii C4 F11 iii (4) H16 C52 viii C5 C (6) H16 H45 vii C5 C (6) H17 C88 viii C6 C (6) H18 C C6 N (4) H18 C C7 O (5) H18 C C8 C102 vii (6) H20 C C9 C102 vii (6) H20 C C12 C (5) H20 C C12 O (4) H21 F1 vii C14 P (3) H21 F2 vii C14 C (5) H22 C78 vii C14 C (4) H22 C75 vii C15 C57 vii (5) H22 C76 vii C15 C (4) H22 C77 vii C15 C (4) H22 C74 vii C15 C58 vii (5) H22 C73 vii C15 C (4) H23 N11 vii C16 C58 vii (5) H26 H41A vi C16 C51 viii (4) H26 C C16 C52 viii (4) H26 N2 vi C17 C88 viii (5) H26 C C18 C (6) H28 C106 iv C19 C (4) H30 N C20 C (5) H30 Cu C20 C (5) H30 N C21 F2 vii (4) H32 C44 vii C21 F1 vii (4) H32 C C23 C (4) H32 C C23 N11 vii (4) H33 C46 vii C26 C (5) H33 C45 vii C26 C35 vi (6) H35 C26 vi C28 C106 iv (5) H35 C25 vi C30 C (5) H35 C28 vi sup-17
19 C31 C (4) H35 C27 vi C32 C41 vi (5) H36 Cu C32 C (4) H37 F C33 C37 vi (5) H37 F C33 N3 vi (4) H40 H C33 C41 vi (5) H40 F C34 C37 vi (5) H40 F5 iv C35 C26 vi (6) H41A H C37 C34 vi (5) H41A H26 vi C37 C33 vi (5) H41A C32 vi C37 F (5) H41B C47 iii C37 F (4) H41B C42 iii C40 F (6) H43 C21 ii C40 F5 iv (4) H43 N C40 C (5) H43 C C41 C32 vi (5) H44 H15 ii C41 C33 vi (5) H45 H16 ii C44 C (6) H46 F4 iii C45 C (6) H46 H48A xi C47 F2 iii (4) H47 F2 iii C48 F6 iv (5) H47 H41A C50 C (5) H48A H C51 C16 ix (4) H48A F6 iv C52 C16 ix (4) H48A H46 xiii C53 F11 iv (5) H48B C104 vii C54 F11 iv (4) H48B H C55 O (4) H50 H48A C57 C15 ii (5) H50 F4 iv C58 C15 ii (5) H50 H C58 C16 ii (5) H50 C C60 C (5) H52 C C60 O (4) H52 C C62 C (6) H52 C C63 C63 x (5) H53 F11 iv C67 C (4) H54 H48B C68 C (6) H54 F11 iv C68 C (4) H54 F8 iv C71 C (4) H56 Cu C71 C (4) H57 H12 ii C72 C (4) H58 C16 ii C72 C (5) H59 F12 iii C72 P (3) H59 F11 iii C73 C (4) H60 C C74 C (5) H60 C C77 C (5) H62 C C77 C (4) H62 C C79 C (4) H62 C99 xi C80 C (5) H62 H99 xi sup-18
20 C81 F12 iv (4) H64 F7 xiv C84 C (4) H64 F10 xiv C84 C (4) H65 F7 xiv C84 N (5) H66 Cu C85 C (5) H66 N C86 C (5) H66 N C86 N (4) H68 C C86 C (5) H68 C C87 C (5) H69 C96 xii C88 C17 ix (5) H69 C97 xii C90 C (5) H70 C C90 C (5) H71 C C92 C (5) H71 C C93 C (5) H71 C C94 F8 i (5) H71 C C94 F9 i (4) H74 C C94 C (5) H74 C C94 C (5) H74 C C1 H H74 C C1 H H76 C C101 C (5) H77 C C101 N (4) H77 C C102 C8 ii (6) H77 C C2 H H77 C C2 H H80 C C102 C9 ii (6) H80 H C3 H H80 C C4 H H81 F8 iv C104 C (5) H81 F12 iv C4 H105 viii H83 H101 xii C5 H105 viii H83 C101 xii C6 H H84 C C106 C28 iv (5) H84 Cu C7 H H84 N C8 H10D vii H86 C C8 H H86 C C9 H H87 C C109 F10 i (11) H87 C C9 H10D vii H87 C C109 F9 i (9) H87 C C11 H H87 C C12 H H87 C C13 H H88 C17 ix C14 H H89 C C16 H58 vii H90 C C17 H88 viii H90 C C19 H H90 C C19 H108 vii H90 C sup-19
21 C20 H108 vii H94 F8 i C20 H H94 P6 i C21 H43 vii H94 F9 i C21 H108 vii H95A C83 xii C22 H108 vii H95B H C23 H H97 H95B C24 H H97 Cl C25 H H98 C62 xiii C25 H35 vi H99 C59 xiii C26 H H99 H62 xiii C26 H35 vi H100 C65 xii C27 H35 vi H100 C C28 H106 iv H101 N C28 H35 vi H101 C C30 H H101 C C31 H H101 H83 xii C32 H41A vi H104 H10D C32 H H104 F9 i C37 H H105 C4 ix C38 H H105 C5 ix C40 H H106 C28 iv C42 H41B iii H107 F C44 H H108 C19 ii C44 H32 ii H108 C20 ii C44 H15 ii H108 C21 ii C45 H H108 C22 ii C45 H33 ii H108 H10E P1 Cu1 P (4) C25 C30 H P1 Cu1 N (7) C33 C32 H P1 Cu1 N (7) C31 C32 H P2 Cu1 N (7) C32 C33 H P2 Cu1 N (8) C34 C33 H N1 Cu1 N (10) C33 C34 H P3 Cu2 N (7) C35 C34 H P4 Cu2 N (7) C36 C35 H P4 Cu2 N (8) C34 C35 H N7 Cu2 N (10) C31 C36 H P3 Cu2 P (4) C35 C36 H P3 Cu2 N (7) C38 C37 H C1 P1 C (15) N3 C37 H Cu1 P1 C (11) N6 C40 H Cu1 P1 C (10) C39 C40 H Cu1 P1 C (11) C42 C41 H41B C1 P1 C (15) C42 C41 H41A C7 P1 C (16) H41A C41 H41B C25 P2 C (14) N3 C41 H41B Cu1 P2 C (10) N3 C41 H41A sup-20
22 Cu1 P2 C (13) C44 C43 H C19 P2 C (16) C42 C43 H C19 P2 C (16) C43 C44 H Cu1 P2 C (13) C45 C44 H C55 P3 C (14) C46 C45 H Cu2 P3 C (10) C44 C45 H Cu2 P3 C (10) C45 C46 H Cu2 P3 C (11) C47 C46 H C55 P3 C (16) C42 C47 H C61 P3 C (16) C46 C47 H Cu2 P4 C (10) N6 C48 H48A Cu2 P4 C (11) N6 C48 H48B Cu2 P4 C (13) C49 C48 H48B C73 P4 C (15) H48A C48 H48B C73 P4 C (13) C49 C48 H48A C79 P4 C (15) C49 C50 H F2 P5 F (15) C51 C50 H F1 P5 F (15) C52 C51 H F1 P5 F (2) C50 C51 H F1 P5 F (2) C53 C52 H F1 P5 F (18) C51 C52 H F1 P5 F (2) C52 C53 H F5 P5 F (2) C54 C53 H F3 P5 F (2) C49 C54 H F3 P5 F (2) C53 C54 H F3 P5 F (18) P3 C55 C (3) F2 P5 F (2) C56 C55 C (3) F4 P5 F (2) P3 C55 C (2) F4 P5 F (2) C55 C56 C (3) F2 P5 F (2) C56 C57 C (4) F2 P5 F (18) C57 C58 C (3) F7 P6 F (14) C58 C59 C (3) F7 P6 F (11) C55 C60 C (4) F11 P6 F (14) P3 C61 C (2) F7 P6 F (14) P3 C61 C (2) F8 P6 F (12) C62 C61 C (3) F8 P6 F (12) C61 C62 C (3) F8 P6 F (11) C62 C63 C (3) F9 P6 F (13) C63 C64 C (3) F7 P6 F (12) C64 C65 C (3) F7 P6 F (13) C61 C66 C (3) F10 P6 F (13) P3 C67 C (3) F9 P6 F (12) P3 C67 C (3) F10 P6 F (11) C68 C67 C (4) F8 P6 F (12) C67 C68 C (3) F9 P6 F (12) C68 C69 C (3) C14 O1 C (2) C69 C70 C (4) C72 O2 C (2) C70 C71 C (3) sup-21
23 Cu1 N1 N (19) O2 C72 C (3) Cu1 N1 C (2) O2 C72 C (3) N2 N1 C (3) C67 C72 C (3) N1 N2 N (2) P4 C73 C (2) N2 N3 C (3) P4 C73 C (2) C37 N3 C (3) C74 C73 C (3) N2 N3 C (3) C73 C74 C (3) N5 N4 C (2) C74 C75 C (3) Cu1 N4 C (18) C75 C76 C (3) Cu1 N4 N (2) C76 C77 C (3) N4 N5 N (2) O2 C78 C (2) N5 N6 C (2) O2 C78 C (2) C40 N6 C (3) C73 C78 C (3) N5 N6 C (2) C80 C79 C (3) Cu2 N7 C (19) P4 C79 C (2) N8 N7 C (2) P4 C79 C (3) Cu2 N7 N (2) C79 C80 C (3) N7 N8 N (2) C80 C81 C (4) C91 N9 C (3) C81 C82 C (3) N8 N9 C (2) C82 C83 C (3) N8 N9 C (3) C79 C84 C (3) Cu2 N10 N (2) P4 C85 C (2) N11 N10 C (3) C86 C85 C (3) Cu2 N10 C (2) P4 C85 C (2) N10 N11 N (2) C85 C86 C (3) C94 N12 C (3) C86 C87 C (3) N11 N12 C (3) C87 C88 C (3) N11 N12 C (3) C88 C89 C (3) P1 C1 C (3) C85 C90 C (3) C2 C1 C (3) N9 C91 C (3) P1 C1 C (2) N7 C92 C (3) C1 C2 C (3) C91 C92 C (3) C2 C3 C (3) N7 C92 C (3) C3 C4 C (3) N10 C93 C (3) C4 C5 C (3) N10 C93 C (3) C1 C6 C (3) C92 C93 C (3) P1 C7 C (2) N12 C94 C (3) P1 C7 C (3) N9 C95 C (3) C8 C7 C (3) C95 C96 C (4) C7 C8 C (3) C95 C96 C (3) C8 C9 C (4) C97 C96 C (4) C9 C10 C (4) C96 C97 C (5) C10 C11 C (4) C97 C98 C (4) C7 C12 C (4) C98 C99 C (5) P1 C13 C (2) C99 C100 C (5) P1 C13 C (3) C96 C101 C (3) C14 C13 C (3) N12 C102 C (3) O1 C14 C (3) C102 C103 C (3) sup-22
24 O1 C14 C (3) C104 C103 C (3) C13 C14 C (3) C102 C103 C (3) C14 C15 C (4) C103 C104 C (4) C15 C16 C (3) C104 C105 C (4) C16 C17 C (3) C105 C106 C (4) C13 C18 C (4) C106 C107 C (4) C20 C19 C (3) C103 C108 C (3) P2 C19 C (2) C57 C56 H P2 C19 C (2) C55 C56 H C19 C20 C (3) C56 C57 H C20 C21 C (3) C58 C57 H C21 C22 C (3) C59 C58 H C22 C23 C (3) C57 C58 H C19 C24 C (3) C58 C59 H O1 C24 C (3) C60 C59 H O1 C24 C (2) C59 C60 H P2 C25 C (2) C55 C60 H C26 C25 C (3) C61 C62 H P2 C25 C (3) C63 C62 H C25 C26 C (4) C64 C63 H C26 C27 C (4) C62 C63 H C27 C28 C (4) C63 C64 H C28 C29 C (4) C65 C64 H C25 C30 C (3) C66 C65 H C32 C31 C (3) C64 C65 H P2 C31 C (3) C61 C66 H P2 C31 C (3) C65 C66 H C31 C32 C (3) C67 C68 H C32 C33 C (4) C69 C68 H C33 C34 C (3) C70 C69 H C34 C35 C (3) C68 C69 H C31 C36 C (4) C71 C70 H N3 C37 C (3) C69 C70 H N1 C38 C (3) C70 C71 H C37 C38 C (3) C72 C71 H N1 C38 C (3) C73 C74 H N4 C39 C (3) C75 C74 H C38 C39 C (3) C76 C75 H N4 C39 C (3) C74 C75 H N6 C40 C (3) C77 C76 H N3 C41 C (3) C75 C76 H C41 C42 C (3) C78 C77 H C43 C42 C (4) C76 C77 H C41 C42 C (3) C79 C80 H C42 C43 C (4) C81 C80 H C43 C44 C (4) C82 C81 H C44 C45 C (4) C80 C81 H C45 C46 C (4) C83 C82 H sup-23
Experimental. Crystal data
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Terephthalic acid 2,2 0 -dimethyl-1,1 0 - (butane-1,4-diyl)dibenzimidazole (2/3) Hui Jiang and Xian-Wu Dong* Department
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Terephthalic acid 2,2 0 -dimethyl-1,1 0 - (butane-1,4-diyl)dibenzimidazole (2/3) Hui Jiang and Xian-Wu Dong* Department
Triclinic, P1 a = (2) Å b = (3) Å c = (4) Å = (1) = (1) = (1) Data collection.
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Benzyl N 0 -(4,6-dimethoxy-2-methyl-3- phenyl-1h-indol-7-ylmethylene)- hydrazinecarbodithioate Hamid Khaldei,
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Benzyl N 0 -(4,6-dimethoxy-2-methyl-3- phenyl-1h-indol-7-ylmethylene)- hydrazinecarbodithioate Hamid Khaldei,
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 [1,2-Bis(1H-benzimidazol-2-yl-jN 3 )- ethane]dichloridozinc(ii) Yan-Ling Zhou, a Ming-Hua Zeng a and Seik
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 [1,2-Bis(1H-benzimidazol-2-yl-jN 3 )- ethane]dichloridozinc(ii) Yan-Ling Zhou, a Ming-Hua Zeng a and Seik
Supplementary Material
 Supplementary Material Synthesis of bis-oxathiaaza[3.3.3]propellanes via nucleophilic addition of (1,ω-alkanediyl)bis(N'-organylthioureas) on dicyanomethylene-1,3-indanedione Alaa A. Hassan, a * Kamal
Supplementary Material Synthesis of bis-oxathiaaza[3.3.3]propellanes via nucleophilic addition of (1,ω-alkanediyl)bis(N'-organylthioureas) on dicyanomethylene-1,3-indanedione Alaa A. Hassan, a * Kamal
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 2,2 0 -Biimidazolium hexaaquamanganese(ii) bis(sulfate) Mukhtar A. Kurawa, Christopher J. Adams and A. Guy
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 2,2 0 -Biimidazolium hexaaquamanganese(ii) bis(sulfate) Mukhtar A. Kurawa, Christopher J. Adams and A. Guy
Synthesis of New Heteroscorpionate Iridium(I) and Iridium(III) Complexes
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 ELECTRONIC SUPPORTING INFORMATION For Synthesis of New Heteroscorpionate Iridium(I)
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 ELECTRONIC SUPPORTING INFORMATION For Synthesis of New Heteroscorpionate Iridium(I)
data reports 2-(4-Methylphenyl)-2-oxoethyl 3,4-dimethoxybenzoate Structure description Synthesis and crystallization Refinement
 2-(4-Methylphenyl)-2-oxoethyl 3,4-dimethoxybenzoate ISSN 2414-3146 Shamantha Kumar, a Chandra, b B. M. Rajesh, c M. Mahendra b and B. H. Doreswamy a * Received 27 December 2016 Accepted 2 February 2017
2-(4-Methylphenyl)-2-oxoethyl 3,4-dimethoxybenzoate ISSN 2414-3146 Shamantha Kumar, a Chandra, b B. M. Rajesh, c M. Mahendra b and B. H. Doreswamy a * Received 27 December 2016 Accepted 2 February 2017
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Triethylammonium bis(oxalato)- oxido(triphenylphosphane)rhenate(v) Philipp Grimminger and Peter Klüfers*
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Triethylammonium bis(oxalato)- oxido(triphenylphosphane)rhenate(v) Philipp Grimminger and Peter Klüfers*
Single Crystal X-Ray Structure Determination of Compounds 8a, 8b and 11a
 Single Crystal X-Ray Structure Determination of Compounds 8a, 8b and 11a General: Preliminary examination and data collection were carried out on an area detecting system (Kappa-CCD; Nonius) using graphite-monochromated
Single Crystal X-Ray Structure Determination of Compounds 8a, 8b and 11a General: Preliminary examination and data collection were carried out on an area detecting system (Kappa-CCD; Nonius) using graphite-monochromated
Experimental. Crystal data. C 37 H 38 N 4 O 6 M r = Monoclinic, P2 1 a = (2) Å b = (7) Å c = (2) Å = 112.
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Pheophorbide b ethyl ester from a chlorella vulgaris dietary supplement Experimental Crystal data C 37 H 38 N
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Pheophorbide b ethyl ester from a chlorella vulgaris dietary supplement Experimental Crystal data C 37 H 38 N
Experimental. Crystal data. C 23 H 27 ClN 2 O M r = Triclinic, P1 a = (3) Å b = (6) Å c = (7) Å = (4) = 80.
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 8-{(E)-[(4-Chlorophenyl)imino]methyl}- 1,1,7,7-tetramethyl-1,2,3,5,6,7-hexahydropyrido[3,2,1-ij]quinolin-9-ol
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 8-{(E)-[(4-Chlorophenyl)imino]methyl}- 1,1,7,7-tetramethyl-1,2,3,5,6,7-hexahydropyrido[3,2,1-ij]quinolin-9-ol
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Pentakis(2-oxo-2,3-dihydropyrimidin-1- ium) di-l 3 -chlorido-tri-l 2 -chlorido-hexachloridotricadmate(ii)
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Pentakis(2-oxo-2,3-dihydropyrimidin-1- ium) di-l 3 -chlorido-tri-l 2 -chlorido-hexachloridotricadmate(ii)
IV. ANHANG 179. Anhang 178
 Anhang 178 IV. ANHANG 179 1. Röntgenstrukturanalysen (Tabellen) 179 1.1. Diastereomer A (Diplomarbeit) 179 1.2. Diastereomer B (Diplomarbeit) 186 1.3. Aldoladdukt 5A 193 1.4. Aldoladdukt 13A 200 1.5. Aldoladdukt
Anhang 178 IV. ANHANG 179 1. Röntgenstrukturanalysen (Tabellen) 179 1.1. Diastereomer A (Diplomarbeit) 179 1.2. Diastereomer B (Diplomarbeit) 186 1.3. Aldoladdukt 5A 193 1.4. Aldoladdukt 13A 200 1.5. Aldoladdukt
Supporting Information
 One-Pot, Three-Component Assembly of Indoloquinolines: Total Synthesis of Isocryptolepine Alexander V. Aksenov,* Dmitrii A. Aksenov, Naila A. Orazova, Nicolai A. Aksenov, Georgii D. Griaznov, Annelise
One-Pot, Three-Component Assembly of Indoloquinolines: Total Synthesis of Isocryptolepine Alexander V. Aksenov,* Dmitrii A. Aksenov, Naila A. Orazova, Nicolai A. Aksenov, Georgii D. Griaznov, Annelise
Enantioselective Synthesis of the Anti-inflammatory Agent ( )-Acanthoic Acid
 Enantioselective Synthesis of the Anti-inflammatory Agent ( )-Acanthoic Acid Taotao Ling, a Chinmay Chowdhury, a Bryan A. Kramer, a Binh G. Vong, a Michael A. Palladino b and Emmanuel A. Theodorakis a
Enantioselective Synthesis of the Anti-inflammatory Agent ( )-Acanthoic Acid Taotao Ling, a Chinmay Chowdhury, a Bryan A. Kramer, a Binh G. Vong, a Michael A. Palladino b and Emmanuel A. Theodorakis a
Cycloaddition of Homochiral Dihydroimidazoles: A 1,3-Dipolar Cycloaddition Route to Optically Active Pyrrolo[1,2-a]imidazoles
![Cycloaddition of Homochiral Dihydroimidazoles: A 1,3-Dipolar Cycloaddition Route to Optically Active Pyrrolo[1,2-a]imidazoles Cycloaddition of Homochiral Dihydroimidazoles: A 1,3-Dipolar Cycloaddition Route to Optically Active Pyrrolo[1,2-a]imidazoles](/thumbs/89/100111450.jpg) X-Ray crystallographic data tables for paper: Supplementary Material (ESI) for Organic & Biomolecular Chemistry Cycloaddition of Homochiral Dihydroimidazoles: A 1,3-Dipolar Cycloaddition Route to Optically
X-Ray crystallographic data tables for paper: Supplementary Material (ESI) for Organic & Biomolecular Chemistry Cycloaddition of Homochiral Dihydroimidazoles: A 1,3-Dipolar Cycloaddition Route to Optically
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 = 91.155 (4) = 108.148 (4) = 111.344 (3) V = 894.09 (7) Å 3 Z =1 Mo K radiation = 17.13 mm 1 T = 295 K 0.41
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 = 91.155 (4) = 108.148 (4) = 111.344 (3) V = 894.09 (7) Å 3 Z =1 Mo K radiation = 17.13 mm 1 T = 295 K 0.41
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2008
 Supporting Information Copyright Wiley-VC Verlag Gmb & Co. KGaA, 69451 Weinheim, 2008 Copper Complexes of Mono- and Ditopic [(Methylthio)methyl]borates: Missing Links and Linked Systems En Route to Copper
Supporting Information Copyright Wiley-VC Verlag Gmb & Co. KGaA, 69451 Weinheim, 2008 Copper Complexes of Mono- and Ditopic [(Methylthio)methyl]borates: Missing Links and Linked Systems En Route to Copper
Supporting Information File 2. Crystallographic data of syn-bis-quinoxaline, 16c CH 3 CO 2 C 2 H 5 ;
 Supporting Information File 2 Crystallographic data of syn-bis-quinoxaline, 16c CH 3 CO 2 C 2 H 5 ; Preparation, structures and host guest chemistry of fluorinated syn-bisquinoxaline molecular tweezers
Supporting Information File 2 Crystallographic data of syn-bis-quinoxaline, 16c CH 3 CO 2 C 2 H 5 ; Preparation, structures and host guest chemistry of fluorinated syn-bisquinoxaline molecular tweezers
Supporting Information
 Supporting Information Vinylogous elimination/heck coupling/allylation domino reactions: access to 2- substituted 2,3-dihydrobenzofurans and indolines Jianguo Yang, *, Hanjie Mo, Xiuxiu Jin, Dongdong Cao,
Supporting Information Vinylogous elimination/heck coupling/allylation domino reactions: access to 2- substituted 2,3-dihydrobenzofurans and indolines Jianguo Yang, *, Hanjie Mo, Xiuxiu Jin, Dongdong Cao,
Nickel and Platinum PCP Pincer Complexes Incorporating an Acyclic Diaminoalkyl Central Moiety Connecting Imidazole or Pyrazole Rings
 ickel and Platinum PCP Pincer Complexes Incorporating an Acyclic Diaminoalkyl Central Moiety Connecting Imidazole or Pyrazole Rings Braulio M. Puerta Lombardi, Rudy M. Braun, Chris Gendy, Chia Yun Chang,
ickel and Platinum PCP Pincer Complexes Incorporating an Acyclic Diaminoalkyl Central Moiety Connecting Imidazole or Pyrazole Rings Braulio M. Puerta Lombardi, Rudy M. Braun, Chris Gendy, Chia Yun Chang,
organic papers 7,8-Dihydroxy-3-methyl-10-oxo-1H,10Hpyrano[4,3-b]chromene-9-carboxylic
![organic papers 7,8-Dihydroxy-3-methyl-10-oxo-1H,10Hpyrano[4,3-b]chromene-9-carboxylic organic papers 7,8-Dihydroxy-3-methyl-10-oxo-1H,10Hpyrano[4,3-b]chromene-9-carboxylic](/thumbs/91/105777620.jpg) organic papers Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 7,8-Dihydroxy-3-methyl-10-oxo-1H,10Hpyrano[4,3-b]chromene-9-carboxylic acid Jian-Feng Wang, a * Yong-Jie Zhang, b
organic papers Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 7,8-Dihydroxy-3-methyl-10-oxo-1H,10Hpyrano[4,3-b]chromene-9-carboxylic acid Jian-Feng Wang, a * Yong-Jie Zhang, b
Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes
![Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes](/thumbs/92/108867714.jpg) SUPPORTING INFORMATION Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Eric J. Uzelac, Casey B. McCausland, and Seth C. Rasmussen*
SUPPORTING INFORMATION Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Eric J. Uzelac, Casey B. McCausland, and Seth C. Rasmussen*
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Cyclopentadienyl iron dicarbonyl (CpFe(CO) 2 ) derivatives
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Cyclopentadienyl iron dicarbonyl (CpFe(CO) 2 ) derivatives
Supplementary information
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2015 Supplementary information Hybrids of acylated homoserine lactone and nitric
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2015 Supplementary information Hybrids of acylated homoserine lactone and nitric
Photo-Induced Self-Assembly of Pt(II)-Linked Rings and Cages via the Photolabilization of a Pt(II) Pyridine Bond
 Photo-Induced Self-Assembly of Pt(II)-Linked Rings and Cages via the Photolabilization of a Pt(II) Pyridine Bond Ken-ichi Yamashita, Kei-ichi Sato, Masaki Kawano and Makoto Fujita* Contents; Figure S1.
Photo-Induced Self-Assembly of Pt(II)-Linked Rings and Cages via the Photolabilization of a Pt(II) Pyridine Bond Ken-ichi Yamashita, Kei-ichi Sato, Masaki Kawano and Makoto Fujita* Contents; Figure S1.
Supporting Information-B. A Facile Iterative Synthesis of 2,5-Terpyrimidinylenes as Non-peptidic α-helical Mimics
 Supporting Information-B A Facile Iterative Synthesis of 2,5-Terpyrimidinylenes as on-peptidic α-helical Mimics Laura Anderson, 1,2 Mingzhou Zhou, 1,2 Vasudha Sharma, 2 Jillian M. McLaughlin, 2 Daniel.
Supporting Information-B A Facile Iterative Synthesis of 2,5-Terpyrimidinylenes as on-peptidic α-helical Mimics Laura Anderson, 1,2 Mingzhou Zhou, 1,2 Vasudha Sharma, 2 Jillian M. McLaughlin, 2 Daniel.
2-Carboxyquinolinium-2,4,6-trinitrobenzenesulfonatequinolinium-2-carboxylate
 2-Carboxyquinolinium-2,4,6-trinitrobenzenesulfonatequinolinium-2-carboxylate (1/1/1) Author Smith, Graham, Wermuth, Urs, M. White, Jonathan Published 2008 Journal Title Acta Crystallographica. Section
2-Carboxyquinolinium-2,4,6-trinitrobenzenesulfonatequinolinium-2-carboxylate (1/1/1) Author Smith, Graham, Wermuth, Urs, M. White, Jonathan Published 2008 Journal Title Acta Crystallographica. Section
Supporting Information. Palladium Complexes with Bulky Diphosphine. Synthesis of (Bio-) Adipic Acid from Pentenoic. Acid Mixtures.
 Supporting Information Palladium Complexes with Bulky Diphosphine Ligands as Highly Selective Catalysts for the Synthesis of (Bio-) Adipic Acid from Pentenoic Acid Mixtures. Choon Heng Low, James D. Nobbs,*
Supporting Information Palladium Complexes with Bulky Diphosphine Ligands as Highly Selective Catalysts for the Synthesis of (Bio-) Adipic Acid from Pentenoic Acid Mixtures. Choon Heng Low, James D. Nobbs,*
Multifunctinality and Crystal Dynamics of Highly Stable Porous Metal-Organic Framework [Zn 4 O(NTB) 2 ]
![Multifunctinality and Crystal Dynamics of Highly Stable Porous Metal-Organic Framework [Zn 4 O(NTB) 2 ] Multifunctinality and Crystal Dynamics of Highly Stable Porous Metal-Organic Framework [Zn 4 O(NTB) 2 ]](/thumbs/92/109256432.jpg) Supporting Information Multifunctinality and Crystal Dynamics of Highly Stable Porous Metal-Organic Framework [Zn 4 O(NTB) 2 ] Eun Young Lee, Seung Yeon Jang, and Myunghyun Paik Suh* School of Chemistry,
Supporting Information Multifunctinality and Crystal Dynamics of Highly Stable Porous Metal-Organic Framework [Zn 4 O(NTB) 2 ] Eun Young Lee, Seung Yeon Jang, and Myunghyun Paik Suh* School of Chemistry,
chlorostibine Iou-Sheng Ke and François P. Gabbai Department of Chemistry, Texas A&M University, College Station, TX
 σ-donor/acceptor confused ligands: The case of a chlorostibine Iou-Sheng Ke and François P. Gabbai Department of Chemistry, Texas A&M University, College Station, TX 77843-3255. *To whom correspondence
σ-donor/acceptor confused ligands: The case of a chlorostibine Iou-Sheng Ke and François P. Gabbai Department of Chemistry, Texas A&M University, College Station, TX 77843-3255. *To whom correspondence
NH-Type of chiral Ni(II) complexes of glycine Schiff base: design, structural evaluation, reactivity and synthetic applications
 Supporting Information NH-Type of chiral Ni(II) complexes of glycine Schiff base: design, structural evaluation, reactivity and synthetic applications Mackenzie Bergagnini, a Kazunobu Fukushi, b Jianlin
Supporting Information NH-Type of chiral Ni(II) complexes of glycine Schiff base: design, structural evaluation, reactivity and synthetic applications Mackenzie Bergagnini, a Kazunobu Fukushi, b Jianlin
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Bis(tetrabutylammonium) bis(3,4,5- trioxocyclopent-1-ene-1,2-dithiolatoj 2 S,S 0 )cadmate(ii) 0.25-hydrate
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Bis(tetrabutylammonium) bis(3,4,5- trioxocyclopent-1-ene-1,2-dithiolatoj 2 S,S 0 )cadmate(ii) 0.25-hydrate
C H Activation of Cp* Ligand Coordinated to Ruthenium. Center: Synthesis and Reactivity of a Thiolate-Bridged
 Supporting Information C H Activation of Cp* Ligand Coordinated to Ruthenium Center: Synthesis and Reactivity of a Thiolate-Bridged Diruthenium Complex Featuring Fulvene-like Cp* Ligand Xiaoxiao Ji, Dawei
Supporting Information C H Activation of Cp* Ligand Coordinated to Ruthenium Center: Synthesis and Reactivity of a Thiolate-Bridged Diruthenium Complex Featuring Fulvene-like Cp* Ligand Xiaoxiao Ji, Dawei
Supporting Information. Pd(0)-Catalyzed Decarboxylative Coupling and Tandem C H Arylation/Decarboxylation for the. Synthesis of Heteroaromatic Biaryls
 Supporting Information Pd()-Catalyzed Decarboxylative Coupling and Tandem C H Arylation/Decarboxylation for the Synthesis of Heteroaromatic Biaryls Debkumar andi, Yang-Ming Jhou, Jhen-Yi Lee, Bing-Chiuan
Supporting Information Pd()-Catalyzed Decarboxylative Coupling and Tandem C H Arylation/Decarboxylation for the Synthesis of Heteroaromatic Biaryls Debkumar andi, Yang-Ming Jhou, Jhen-Yi Lee, Bing-Chiuan
Patrycja Miszczyk, Dorota Wieczorek, Joanna Gałęzowska, Błażej Dziuk, Joanna Wietrzyk and Ewa Chmielewska. 1. Spectroscopic Data.
 ; doi:10.3390/molecules22020254 S1 of S23 Supplementary Materials: Reaction of 3-Amino-1,2,4-Triazole with Diethyl Phosphite and Triethyl Orthoformate: Acid-Base Properties and Antiosteoporotic Activities
; doi:10.3390/molecules22020254 S1 of S23 Supplementary Materials: Reaction of 3-Amino-1,2,4-Triazole with Diethyl Phosphite and Triethyl Orthoformate: Acid-Base Properties and Antiosteoporotic Activities
Experimental. Crystal data. C 37 H 24 N 2 O 3 M r = Orthorhombic, P a = (1) Å b = (2) Å c = 26.
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 1,3-Bis(naphthalen-2-ylmethyl)-1H- anthra[1,2-d]imidazole-2,6,11(3h)- trione Zahra Afrakssou, a Youssef Kandri
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 1,3-Bis(naphthalen-2-ylmethyl)-1H- anthra[1,2-d]imidazole-2,6,11(3h)- trione Zahra Afrakssou, a Youssef Kandri
2. Experimental Crystal data. C 22 H 29 F 3 O 5 S M r = Orthorhombic, P a = (10) Å b = (12) Å c = 27.
 data reports ISSN 2056-9890 Crystal structure of 3b-acetoxyandrosta- 5,16-dien-17-yl trifluoromethanesulfonate Shengjun Zhou, a Huaqi Huang, b Ting Zhang, b Dangfeng Wang b and Rongbin Huang b * a Hangzhou
data reports ISSN 2056-9890 Crystal structure of 3b-acetoxyandrosta- 5,16-dien-17-yl trifluoromethanesulfonate Shengjun Zhou, a Huaqi Huang, b Ting Zhang, b Dangfeng Wang b and Rongbin Huang b * a Hangzhou
Supporting Information
 Supporting Information for Pyrene nucleobase conjugates: synthesis, oligonucleotide binding and confocal bioimaging studies Artur Jabłoński 1, Yannic Fritz 2, Hans-Achim Wagenknecht 2, Rafał Czerwieniec
Supporting Information for Pyrene nucleobase conjugates: synthesis, oligonucleotide binding and confocal bioimaging studies Artur Jabłoński 1, Yannic Fritz 2, Hans-Achim Wagenknecht 2, Rafał Czerwieniec
Table of Contents 1 Supplementary Data MCD
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) CPh 3 as a functional group in P-heterocyclic
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) CPh 3 as a functional group in P-heterocyclic
Supporting Information
 Supporting Information 1,3,5-Triazapentadienes by Nucleophilic Addition to 1,3- and 1,4-Dinitriles - Sterically Constrained Examples by Incorporation into Cyclic Peripheries: Synthesis, Aggregation and
Supporting Information 1,3,5-Triazapentadienes by Nucleophilic Addition to 1,3- and 1,4-Dinitriles - Sterically Constrained Examples by Incorporation into Cyclic Peripheries: Synthesis, Aggregation and
Zebra reaction or the recipe for heterodimeric zinc complexes synthesis
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 05 Supporting Information Zebra reaction or the recipe for heterodimeric zinc complexes synthesis
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 05 Supporting Information Zebra reaction or the recipe for heterodimeric zinc complexes synthesis
Supporting Information. Research Center for Marine Drugs, Department of Pharmacy, State Key Laboratory
 Supporting Information Dysiherbols A C and Dysideanone E, Cytotoxic and NF-κB Inhibitory Tetracyclic Meroterpenes from a Dysidea sp. Marine Sponge Wei-Hua Jiao,, Guo-Hua Shi,, Ting-Ting Xu,, Guo-Dong Chen,
Supporting Information Dysiherbols A C and Dysideanone E, Cytotoxic and NF-κB Inhibitory Tetracyclic Meroterpenes from a Dysidea sp. Marine Sponge Wei-Hua Jiao,, Guo-Hua Shi,, Ting-Ting Xu,, Guo-Dong Chen,
Mouhamadou Birame Diop, a * Libasse Diop a and Allen G. Oliver b
 Dimethyl N-cyanodithioiminocarbonate data reports ISSN 2414-3146 Mouhamadou Birame Diop, a * Libasse Diop a and Allen G. Oliver b a Laboratoire de Chimie Minérale et Analytique, Département de Chimie,
Dimethyl N-cyanodithioiminocarbonate data reports ISSN 2414-3146 Mouhamadou Birame Diop, a * Libasse Diop a and Allen G. Oliver b a Laboratoire de Chimie Minérale et Analytique, Département de Chimie,
SUPPORTING INFORMATION. Diastereoselective synthesis of nitroso acetals from (S,E)- -aminated
 SUPPORTING INFORMATION for Diastereoselective synthesis of nitroso acetals from (S,E)- -aminated nitroalkenes via multicomponent [4 + 2]/[3 + 2] cycloadditions promoted by LiCl or LiClO 4 Leandro Lara
SUPPORTING INFORMATION for Diastereoselective synthesis of nitroso acetals from (S,E)- -aminated nitroalkenes via multicomponent [4 + 2]/[3 + 2] cycloadditions promoted by LiCl or LiClO 4 Leandro Lara
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Chlorido(chlorodiphenylphosphine-jP)- (diphenylpiperidinophosphine-jp)- (g 5 -pentamethylcyclopentadienyl)-
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Chlorido(chlorodiphenylphosphine-jP)- (diphenylpiperidinophosphine-jp)- (g 5 -pentamethylcyclopentadienyl)-
data reports a molecular salt Structure description
 ISSN 2414-3146 N,N,N-Triethylethanaminium 5,11,17,23-tetra-tertbutyl-25-[(ethoxycarbonyl)methoxy]-26,28-dihydroxy-27-oxido-2,8,14,20-tetrathiacalix[4]arene: a molecular salt Received 14 September 2016
ISSN 2414-3146 N,N,N-Triethylethanaminium 5,11,17,23-tetra-tertbutyl-25-[(ethoxycarbonyl)methoxy]-26,28-dihydroxy-27-oxido-2,8,14,20-tetrathiacalix[4]arene: a molecular salt Received 14 September 2016
2. Experimental. 2.1. Crystal data
 data reports ISSN 2056-9890 Crystal structure of N-[(morpholin-4- yl)(thiophen-2-yl)methyl]benzamide S. Arun Prabhu, a M. Suresh, b A. Abdul Jameel, c M. Syed Ali Padusha b and B. Gunasekaran d * a Department
data reports ISSN 2056-9890 Crystal structure of N-[(morpholin-4- yl)(thiophen-2-yl)methyl]benzamide S. Arun Prabhu, a M. Suresh, b A. Abdul Jameel, c M. Syed Ali Padusha b and B. Gunasekaran d * a Department
Supporting information. An unusual bifunctional Tb-MOF for highly sensing of Ba 2+ ions and remarkable selectivities of CO 2 /N 2 and CO 2 /CH 4
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information An unusual bifunctional Tb-MOF for highly sensing
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information An unusual bifunctional Tb-MOF for highly sensing
metal-organic compounds
 metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 [l-bis(diphenylarsino)methane- 1:2j 2 As:As 0 ]nonacarbonyl- 1j 3 C,2j 3 C,3j 3 C-tricyclohexylphosphine-
metal-organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 [l-bis(diphenylarsino)methane- 1:2j 2 As:As 0 ]nonacarbonyl- 1j 3 C,2j 3 C,3j 3 C-tricyclohexylphosphine-
Electronic supplementary information (ESI) Bodipy functionalized ortho-carborane dyads for low-energy photosensitization
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information (ESI) Bodipy functionalized ortho-carborane dyads
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information (ESI) Bodipy functionalized ortho-carborane dyads
Enantioselective Organocatalytic Michael Addition of Isorhodanines. to α, β-unsaturated Aldehydes
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Supporting Information
 SUPPORTING INFORMATION FOR: Trialkylstibine complexes of boron, aluminium, gallium and indium trihalides: synthesis, properties and bonding Victoria K. Greenacre, William Levason and Gillian Reid Chemistry,
SUPPORTING INFORMATION FOR: Trialkylstibine complexes of boron, aluminium, gallium and indium trihalides: synthesis, properties and bonding Victoria K. Greenacre, William Levason and Gillian Reid Chemistry,
Supplementary Information. Living Ring-Opening Polymerization of Lactones by N-Heterocyclic Olefin/Al(C 6 F 5 ) 3
 Supplementary Information Living Ring-Opening Polymerization of Lactones by N-Heterocyclic Olefin/Al(C 6 F 5 ) 3 Lewis Pairs: Structures of Intermediates, Kinetics, and Mechanism Qianyi Wang, Wuchao Zhao,
Supplementary Information Living Ring-Opening Polymerization of Lactones by N-Heterocyclic Olefin/Al(C 6 F 5 ) 3 Lewis Pairs: Structures of Intermediates, Kinetics, and Mechanism Qianyi Wang, Wuchao Zhao,
Supporting Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting Information Domino reaction of cyclic sulfamidate imines with
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting Information Domino reaction of cyclic sulfamidate imines with
Tunable Ligand Emission of Napthylsalophen Triple-Decker Dinuclear Lanthanide (III) Sandwich Complexes
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supplemental Information Tunable Ligand Emission of Napthylsalophen Triple-Decker Dinuclear
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supplemental Information Tunable Ligand Emission of Napthylsalophen Triple-Decker Dinuclear
Nitric oxide (NO) reactivity studies on mononuclear Iron(II) complexes supported by a tetradentate Schiff base Ligand
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Nitric oxide (NO) reactivity studies on mononuclear Iron(II) complexes supported by a tetradentate
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Nitric oxide (NO) reactivity studies on mononuclear Iron(II) complexes supported by a tetradentate
2-(5-Bromo-1H-indol-3-yl)-4-(4-bromophenyl)-5- (4-chlorobenzoyl)-1H-pyrrole-3-carbonitrile dimethyl sulfoxide monosolvate
 ISSN 2414-3146 2-(5-Bromo-1H-indol-3-yl)-4-(4-bromophenyl)-5- (4-chlorobenzoyl)-1H-pyrrole-3-carbonitrile dimethyl sulfoxide monosolvate Y. AaminaNaaz, a Jayabal Kamalraja, b G. Vimala, a Paramasivam T.
ISSN 2414-3146 2-(5-Bromo-1H-indol-3-yl)-4-(4-bromophenyl)-5- (4-chlorobenzoyl)-1H-pyrrole-3-carbonitrile dimethyl sulfoxide monosolvate Y. AaminaNaaz, a Jayabal Kamalraja, b G. Vimala, a Paramasivam T.
Supporting Information
 Supporting Information Wiley-VCH 27 69451 Weinheim, Germany Supplementary Figure 1. Synthetic results as detected by XRD (Cu-Kα). Simulation pattern of MCM-68 Relative Intensity / a.u. YNU-2P Conventional
Supporting Information Wiley-VCH 27 69451 Weinheim, Germany Supplementary Figure 1. Synthetic results as detected by XRD (Cu-Kα). Simulation pattern of MCM-68 Relative Intensity / a.u. YNU-2P Conventional
SUPPORTING INFORMATION. Pyramidanes: The Covalent Form of the Ionic Compounds
 SUPPORTING INFORMATION Pyramidanes: The Covalent Form of the Ionic Compounds Vladimir Ya. Lee, 1 * Olga A. Gapurenko, 2 Yuki Ito, 1 Takahiko Meguro, 1 Haruka Sugasawa, 1 Akira Sekiguchi, 1 *, Ruslan M.
SUPPORTING INFORMATION Pyramidanes: The Covalent Form of the Ionic Compounds Vladimir Ya. Lee, 1 * Olga A. Gapurenko, 2 Yuki Ito, 1 Takahiko Meguro, 1 Haruka Sugasawa, 1 Akira Sekiguchi, 1 *, Ruslan M.
Experimental. Crystal data. C 15 H 12 BrN 3 O 2 S M r = Trigonal, R3 a = (9) Å c = (5) Å V = (4) Å 3.
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 N-[(2S)-2-(4-Bromophenyl)-4-oxo-1,3- thiazolidin-3-yl]pyridine-3-carboxamide Mehmet Akkurt, a *Ísmail Çelik, b
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 N-[(2S)-2-(4-Bromophenyl)-4-oxo-1,3- thiazolidin-3-yl]pyridine-3-carboxamide Mehmet Akkurt, a *Ísmail Çelik, b
Supplementary Materials: Exploration of vanadate selenites Solid Phase Space, crystal structures and polymorphism
 Supplementary Materials: Exploration of vanadate selenites Solid Phase Space, crystal structures and polymorphism Vadim M. Kovrugin a,b, Marie Colmont a, Oleg I. Siidra b, Sergey V. Krivovichev b, Olivier
Supplementary Materials: Exploration of vanadate selenites Solid Phase Space, crystal structures and polymorphism Vadim M. Kovrugin a,b, Marie Colmont a, Oleg I. Siidra b, Sergey V. Krivovichev b, Olivier
Supporting Information for. Catalytic C H α-trifluoromethylation of α,β-unsaturated Carbonyl Compounds
 Supporting Information for Catalytic C H α-trifluoromethylation of α,β-unsaturated Carbonyl Compounds Zhongxue Fang, a Yongquan Ning, a Pengbing Mi, a Peiqiu Liao, a Xihe Bi* a,b a Department of Chemistry,
Supporting Information for Catalytic C H α-trifluoromethylation of α,β-unsaturated Carbonyl Compounds Zhongxue Fang, a Yongquan Ning, a Pengbing Mi, a Peiqiu Liao, a Xihe Bi* a,b a Department of Chemistry,
Table S1. Summary of data collections and structure refinements for crystals 1Rb-1h, 1Rb-2h, and 1Rb-4h.
 Supporting Information [NH 3 CH 3 ] [In SbS 9 SH]: A novel methylamine-directed indium thioantimonate with Rb + ion-exchange property Kai-Yao Wang a,b, Mei-Ling Feng a, Jian-Rong Li a and Xiao-Ying Huang
Supporting Information [NH 3 CH 3 ] [In SbS 9 SH]: A novel methylamine-directed indium thioantimonate with Rb + ion-exchange property Kai-Yao Wang a,b, Mei-Ling Feng a, Jian-Rong Li a and Xiao-Ying Huang
Stereochemistry and mechanistic insight in the [2 k +2 i +2 i ] annulations of ketenes and imines
![Stereochemistry and mechanistic insight in the [2 k +2 i +2 i ] annulations of ketenes and imines Stereochemistry and mechanistic insight in the [2 k +2 i +2 i ] annulations of ketenes and imines](/thumbs/83/87678994.jpg) Stereochemistry and mechanistic insight in the [2 k +2 i +2 i ] annulations of ketenes and imines Zhanhui Yang, Wei He, Baoxiang Cheng and Jiaxi Xu* State Key Laboratory of Chemical Resource Engineering,
Stereochemistry and mechanistic insight in the [2 k +2 i +2 i ] annulations of ketenes and imines Zhanhui Yang, Wei He, Baoxiang Cheng and Jiaxi Xu* State Key Laboratory of Chemical Resource Engineering,
Synthesis, Crystal Structure and Supramolecular Understanding of 1,3,5-Tris(1-phenyl-1H-pyrazol-5- yl)benzenes
 Supplementary information Synthesis, Crystal Structure and Supramolecular Understanding of 1,3,5-Tris(1-phenyl-1H-pyrazol-5- yl)benzenes Marcos A. P. Martins 1 *, Alexandre R. Meyer 1, Paulo R. S. Salbego
Supplementary information Synthesis, Crystal Structure and Supramolecular Understanding of 1,3,5-Tris(1-phenyl-1H-pyrazol-5- yl)benzenes Marcos A. P. Martins 1 *, Alexandre R. Meyer 1, Paulo R. S. Salbego
Supporting Information. Generation Response. Physics & Chemistry of CAS, 40-1 South Beijing Road, Urumqi , China. China , USA
 Supporting Information Pb 3 B 6 O 11 F 2 : A First Noncentrocentric Lead Fluoroborate with Large Second Harmonic Generation Response Hongyi Li, a Hongping Wu, a * Xin Su, a Hongwei Yu, a,b Shilie Pan,
Supporting Information Pb 3 B 6 O 11 F 2 : A First Noncentrocentric Lead Fluoroborate with Large Second Harmonic Generation Response Hongyi Li, a Hongping Wu, a * Xin Su, a Hongwei Yu, a,b Shilie Pan,
Supporting Information
 Supporting Information On the Ambiguity of 1,3,2-Benzodiazaboroles as Donor/Acceptor unctionalities in Luminescent Molecules Lothar Weber *[a], Johannes Halama [a], Kenny Hanke [a], Lena Böhling [a], Andreas
Supporting Information On the Ambiguity of 1,3,2-Benzodiazaboroles as Donor/Acceptor unctionalities in Luminescent Molecules Lothar Weber *[a], Johannes Halama [a], Kenny Hanke [a], Lena Böhling [a], Andreas
ANNEXE 2 : SPECTRES DE RÉSONANCE MAGNÉTIQUE NUCLÉAIRE
 ANNEXE 2 : SPECTRES DE RÉSONANCE MAGNÉTIQUE NUCLÉAIRE 175 4-Oxopentyl acetate (1-9) 27.56 17.91 63.49 39.76 29.86 22.66 2.82 2. 1.97 2.91 2.96 2.9 4. 3.98 3.96 2.47 2.44 2.42 2.7 1.95 1.86 1.84 1.82 1.79
ANNEXE 2 : SPECTRES DE RÉSONANCE MAGNÉTIQUE NUCLÉAIRE 175 4-Oxopentyl acetate (1-9) 27.56 17.91 63.49 39.76 29.86 22.66 2.82 2. 1.97 2.91 2.96 2.9 4. 3.98 3.96 2.47 2.44 2.42 2.7 1.95 1.86 1.84 1.82 1.79
Supporting Information
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information Phosphorescent Pt(II) complexes bearing a monoanionic C^N^N luminophore
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information Phosphorescent Pt(II) complexes bearing a monoanionic C^N^N luminophore
Supporting Information
 Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
organic compounds 78 HeÂctor Novoa de Armas et al. C 24 H 36 O 6 Acta Cryst. (2000). C56, 78±79 Comment Experimental
 organic compounds Acta Crystallographica Section C Crystal Structure Communications ISSN 0108-2701 In connection with our studies on the synthesis and characterization of bioactive steroids, we determined
organic compounds Acta Crystallographica Section C Crystal Structure Communications ISSN 0108-2701 In connection with our studies on the synthesis and characterization of bioactive steroids, we determined
Table S1 Selected bond lengths [Å] and angles [ ] for complexes 1 8. Complex 1. Complex 2. Complex 3. Complex 4. Complex 5.
![Table S1 Selected bond lengths [Å] and angles [ ] for complexes 1 8. Complex 1. Complex 2. Complex 3. Complex 4. Complex 5. Table S1 Selected bond lengths [Å] and angles [ ] for complexes 1 8. Complex 1. Complex 2. Complex 3. Complex 4. Complex 5.](/thumbs/65/53614116.jpg) Table S1 Selected bond lengths [Å] and angles [ ] for complexes 1 8. Complex 1 Zn(1) O(1) 1.969(2) Zn(1) O(5) 1.989(3) Zn(1) O(6) 1.930(3) Zn(1) O(4) a 1.954(4) Zn(1) O(3) a 2.519(25) O(6) Zn(1) O(4) a
Table S1 Selected bond lengths [Å] and angles [ ] for complexes 1 8. Complex 1 Zn(1) O(1) 1.969(2) Zn(1) O(5) 1.989(3) Zn(1) O(6) 1.930(3) Zn(1) O(4) a 1.954(4) Zn(1) O(3) a 2.519(25) O(6) Zn(1) O(4) a
Direct metallation of thienopyrimidines using a mixed lithium-cadmium base and antitumor activity of functionalized derivatives
 Direct metallation of thienopyrimidines using a mixed lithium-cadmium base and antitumor activity of functionalized derivatives Katia négaroff, a Frédéric Lassagne, a Ghenia Bentabed-Ababsa, a,b Ekhlass
Direct metallation of thienopyrimidines using a mixed lithium-cadmium base and antitumor activity of functionalized derivatives Katia négaroff, a Frédéric Lassagne, a Ghenia Bentabed-Ababsa, a,b Ekhlass
Supplementary Information for
 Supplentary Information for Aggregation induced blue-shifted ission molecular picture from QM/MM study Qunyan Wu, a Tian Zhang, a Qian Peng,* b Dong Wang, a and Zhigang Shuai* a a Key Loratory of Organic
Supplentary Information for Aggregation induced blue-shifted ission molecular picture from QM/MM study Qunyan Wu, a Tian Zhang, a Qian Peng,* b Dong Wang, a and Zhigang Shuai* a a Key Loratory of Organic
Electronic Supporting Information. 3-Aminothiophenecarboxylic acid (3-Atc)-induced folding in peptides
 Electronic Supplementary Material (ESI for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 Electronic Supporting Information
Electronic Supplementary Material (ESI for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 Electronic Supporting Information
Elizabeth M. Horstman, a Jeffery A. Bertke, b Toby J. Woods b * and Paul J. A. Kenis a
 research communications ISSN 2056-9890 Crystal structure of a 2:1 piroxicam gentisic acid co-crystal featuring neutral and zwitterionic piroxicam molecules Elizabeth M. Horstman, a Jeffery A. Bertke, b
research communications ISSN 2056-9890 Crystal structure of a 2:1 piroxicam gentisic acid co-crystal featuring neutral and zwitterionic piroxicam molecules Elizabeth M. Horstman, a Jeffery A. Bertke, b
Supporting Information
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Supporting Information Abnormal N-Heterocyclic Carbene Based Nickel Complex for Catalytic
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Supporting Information Abnormal N-Heterocyclic Carbene Based Nickel Complex for Catalytic
Bloco A, Cidade Universitária, Ilha do Fundão, Rio de Janeiro, RJ, Brazil. Contents Pages
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Copper(II) Catalyzed Synthesis of Novel Helical
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Copper(II) Catalyzed Synthesis of Novel Helical
Experimental. Crystal data
 organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Enrofloxacin hydrochloride dihydrate Jorge E. Miranda-Calderón, a Lilia Gutiérrez, a Marcos Flores-Alamo, b Ponciano
organic compounds Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 Enrofloxacin hydrochloride dihydrate Jorge E. Miranda-Calderón, a Lilia Gutiérrez, a Marcos Flores-Alamo, b Ponciano
Electronic Supplementary Information:
 Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Synthesis and Biological Evaluation of Novel Acyclic and Cyclic Glyoxamide derivatives as Bacterial Quorum Sensing and Biofilm Inhibitors
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Synthesis and Biological Evaluation of Novel Acyclic and Cyclic Glyoxamide
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Synthesis and Biological Evaluation of Novel Acyclic and Cyclic Glyoxamide
Supporting Information
 Supporting Information Molecular Cage Impregnated Palladium Nanoparticles: Efficient, Additive-Free Heterogeneous Catalysts for Cyanation of Aryl Halides. Bijnaneswar Mondal, Koushik Acharyya, Prodip Howlader
Supporting Information Molecular Cage Impregnated Palladium Nanoparticles: Efficient, Additive-Free Heterogeneous Catalysts for Cyanation of Aryl Halides. Bijnaneswar Mondal, Koushik Acharyya, Prodip Howlader
College of Life Science, Dalian Nationalities University, Dalian , PR China.
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 Postsynthetic modification
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2018 Postsynthetic modification
Mean bond enthalpy Standard enthalpy of formation Bond N H N N N N H O O O
 Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Resurvey of Possible Seismic Fissures in the Old-Edo River in Tokyo
 Bull. Earthq. Res. Inst. Univ. Tokyo Vol. 2.,**3 pp.,,3,.* * +, -. +, -. Resurvey of Possible Seismic Fissures in the Old-Edo River in Tokyo Kunihiko Shimazaki *, Tsuyoshi Haraguchi, Takeo Ishibe +, -.
Bull. Earthq. Res. Inst. Univ. Tokyo Vol. 2.,**3 pp.,,3,.* * +, -. +, -. Resurvey of Possible Seismic Fissures in the Old-Edo River in Tokyo Kunihiko Shimazaki *, Tsuyoshi Haraguchi, Takeo Ishibe +, -.
FORMULAS FOR STATISTICS 1
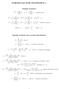 FORMULAS FOR STATISTICS 1 X = 1 n Sample statistics X i or x = 1 n x i (sample mean) S 2 = 1 n 1 s 2 = 1 n 1 (X i X) 2 = 1 n 1 (x i x) 2 = 1 n 1 Xi 2 n n 1 X 2 x 2 i n n 1 x 2 or (sample variance) E(X)
FORMULAS FOR STATISTICS 1 X = 1 n Sample statistics X i or x = 1 n x i (sample mean) S 2 = 1 n 1 s 2 = 1 n 1 (X i X) 2 = 1 n 1 (x i x) 2 = 1 n 1 Xi 2 n n 1 X 2 x 2 i n n 1 x 2 or (sample variance) E(X)
Experimental. Crystal data. C 13 H 17 N 3 O 2 M r = Monoclinic, Cc a = (4) Å b = (4) Å c = (3) Å = 111.
 Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 4-[2-(4-Hydroxyphenyl)ethyl]-3-propyl- 1H-1,2,4-triazol-5(4H)-one Experimental Crystal data C 13 H 17 N 3 O 2 M r = 247.30 Monoclinic,
Acta Crystallographica Section E Structure Reports Online ISSN 1600-5368 4-[2-(4-Hydroxyphenyl)ethyl]-3-propyl- 1H-1,2,4-triazol-5(4H)-one Experimental Crystal data C 13 H 17 N 3 O 2 M r = 247.30 Monoclinic,
Synthesis, Characterization, and Computational Study of Three-Coordinate SNS-Copper(I) Complexes Based on Bis-Thione Precursors
 For Synthesis, Characterization, and Computational Study of Three-Coordinate SNS-Copper(I) Complexes Based on Bis-Thione Precursors John R. Miecznikowski a *; Matthew A. Lynn b ; Jerry P. Jasinski c ;
For Synthesis, Characterization, and Computational Study of Three-Coordinate SNS-Copper(I) Complexes Based on Bis-Thione Precursors John R. Miecznikowski a *; Matthew A. Lynn b ; Jerry P. Jasinski c ;
Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative cleavage of cyclic ethers. Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative
Jesse Maassen and Mark Lundstrom Purdue University November 25, 2013
 Notes on Average Scattering imes and Hall Factors Jesse Maassen and Mar Lundstrom Purdue University November 5, 13 I. Introduction 1 II. Solution of the BE 1 III. Exercises: Woring out average scattering
Notes on Average Scattering imes and Hall Factors Jesse Maassen and Mar Lundstrom Purdue University November 5, 13 I. Introduction 1 II. Solution of the BE 1 III. Exercises: Woring out average scattering
«ΑΓΡΟΤΟΥΡΙΣΜΟΣ ΚΑΙ ΤΟΠΙΚΗ ΑΝΑΠΤΥΞΗ: Ο ΡΟΛΟΣ ΤΩΝ ΝΕΩΝ ΤΕΧΝΟΛΟΓΙΩΝ ΣΤΗΝ ΠΡΟΩΘΗΣΗ ΤΩΝ ΓΥΝΑΙΚΕΙΩΝ ΣΥΝΕΤΑΙΡΙΣΜΩΝ»
 I ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΣΧΟΛΗ ΝΟΜΙΚΩΝ ΟΙΚΟΝΟΜΙΚΩΝ ΚΑΙ ΠΟΛΙΤΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΤΜΗΜΑ ΟΙΚΟΝΟΜΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΣΤΗΝ «ΔΙΟΙΚΗΣΗ ΚΑΙ ΟΙΚΟΝΟΜΙΑ» ΚΑΤΕΥΘΥΝΣΗ: ΟΙΚΟΝΟΜΙΚΗ
I ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΣΧΟΛΗ ΝΟΜΙΚΩΝ ΟΙΚΟΝΟΜΙΚΩΝ ΚΑΙ ΠΟΛΙΤΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΤΜΗΜΑ ΟΙΚΟΝΟΜΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΣΤΗΝ «ΔΙΟΙΚΗΣΗ ΚΑΙ ΟΙΚΟΝΟΜΙΑ» ΚΑΤΕΥΘΥΝΣΗ: ΟΙΚΟΝΟΜΙΚΗ
Electronic, Crystal Chemistry, and Nonlinear Optical Property Relationships. or W, and D = P or V)
 Electronic, Crystal Chemistry, and Nonlinear Optical Property Relationships in the Dugganite A 3 B 3 CD 2 O 14 Family (A = Sr, Ba or Pb; B = Mg or Zn; C = Te or W, and D = P or V) Hongwei Yu, Joshua Young,
Electronic, Crystal Chemistry, and Nonlinear Optical Property Relationships in the Dugganite A 3 B 3 CD 2 O 14 Family (A = Sr, Ba or Pb; B = Mg or Zn; C = Te or W, and D = P or V) Hongwei Yu, Joshua Young,
υγεία των νοσηλευτών που συστηματικά εμπλέκονται στην παρασκευή και χορήγηση τους.
 ΠΕΡΙΛΗΨΗ Εισαγωγή: Τα χημειοθεραπευτικά φάρμακα έχουν αποδειχθεί οτι θέτουν σε κίνδυνο την υγεία των νοσηλευτών που συστηματικά εμπλέκονται στην παρασκευή και χορήγηση τους. Σκοπός: Σκοπός της παρούσας
ΠΕΡΙΛΗΨΗ Εισαγωγή: Τα χημειοθεραπευτικά φάρμακα έχουν αποδειχθεί οτι θέτουν σε κίνδυνο την υγεία των νοσηλευτών που συστηματικά εμπλέκονται στην παρασκευή και χορήγηση τους. Σκοπός: Σκοπός της παρούσας
(M = Mn, Fe, Co, Ni, Cu and Zn)----Mimicking the M II - Substituted Quercetin 2,3-Dioxygenase
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 215 Supplementary material Catalytic Dioxygenation of Flavonol by M II Complexes (M = Mn,
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 215 Supplementary material Catalytic Dioxygenation of Flavonol by M II Complexes (M = Mn,
ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΤΜΗΜΑ ΝΟΣΗΛΕΥΤΙΚΗΣ
 ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΤΜΗΜΑ ΝΟΣΗΛΕΥΤΙΚΗΣ Πτυχιακή εργασία ΓΝΩΣΕΙΣ ΚΑΙ ΣΤΑΣΕΙΣ ΝΟΣΗΛΕΥΤΩΝ ΠΡΟΣ ΤΟΥΣ ΦΟΡΕΙΣ ΜΕ ΣΥΝΔΡΟΜΟ ΕΠΙΚΤΗΤΗΣ ΑΝΟΣΟΑΝΕΠΑΡΚΕΙΑΣ (AIDS) Αλέξης Δημήτρη Α.Φ.Τ: 20085675385 Λεμεσός
ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΤΜΗΜΑ ΝΟΣΗΛΕΥΤΙΚΗΣ Πτυχιακή εργασία ΓΝΩΣΕΙΣ ΚΑΙ ΣΤΑΣΕΙΣ ΝΟΣΗΛΕΥΤΩΝ ΠΡΟΣ ΤΟΥΣ ΦΟΡΕΙΣ ΜΕ ΣΥΝΔΡΟΜΟ ΕΠΙΚΤΗΤΗΣ ΑΝΟΣΟΑΝΕΠΑΡΚΕΙΑΣ (AIDS) Αλέξης Δημήτρη Α.Φ.Τ: 20085675385 Λεμεσός
Four- and Five-membered Cobaltacycles by Regioselective Cyclometalation. of Benzylsulfide Derivatives via Co(V) intermediates
 Electronic Supplementary Information for Dalton Transactions This journal is The Royal Society of Chemistry 2008 Supporting Information for: Four- and Five-membered Cobaltacycles by Regioselective Cyclometalation
Electronic Supplementary Information for Dalton Transactions This journal is The Royal Society of Chemistry 2008 Supporting Information for: Four- and Five-membered Cobaltacycles by Regioselective Cyclometalation
Fused Bis-Benzothiadiazoles as Electron Acceptors
 Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Engineering Tunable Single and Dual Optical. Emission from Ru(II)-Polypyridyl Complexes. Through Excited State Design
 Engineering Tunable Single and Dual Optical Emission from Ru(II)-Polypyridyl Complexes Through Excited State Design Supplementary Information Julia Romanova 1, Yousif Sadik 1, M. R. Ranga Prabhath 1,,
Engineering Tunable Single and Dual Optical Emission from Ru(II)-Polypyridyl Complexes Through Excited State Design Supplementary Information Julia Romanova 1, Yousif Sadik 1, M. R. Ranga Prabhath 1,,
