Towards extracellular Ca 2+ sensing by MRI: synthesis and calciumdependent. ligands
|
|
|
- Τύχων Μέλιοι
- 7 χρόνια πριν
- Προβολές:
Transcript
1 Towards extracellular Ca 2+ sensing by MRI: synthesis and calciumdependent 1 H and 17 O relaxation studies of two novel bismacrocyclic ligands Kirti Dhingra, a Petra Fousková, b Goran ngelovski, a Martin E. Maier, c Nikos K. Logothetis, a,d Éva Tóth* b a Max Planck Institute for Biological Cybernetics, Tübingen, Germany b Centre de Biophysique Moléculaire, CNRS, Orléans Cedex 2, France c Institute für Organische Chemie, Universität Tübingen, Germany d Imaging Science and Biomedical Engineering, University of Manchester, Manchester, U.K. ppendix. Equations used in the analysis of 17 O NMR and 1 H NMRD data. Table S1. Proton relaxivities (r 1 / mm -1 s -1 ) of Gd 2 L 1 in the absence of Ca 2+, c(gd 3+ )=5.1 mm, ph=6.25, at 25 and 37 C. Table S2. Proton relaxivities (r 1 / mm -1 s -1 ) of Gd 2 L 1 in the presence of Ca 2+, c(gd 3+ )=3.2 mm, c(ca 2+ )=4.4 mm, ph=7, at 15.5, 25 and 37 C. Table S3. Variable temperature reduced transverse and longitudinal 17 O relaxation rates of Gd 2 L 1 in the absence of Ca 2+, c(gd 3+ )=51.8 mm, ph=7, P m = at T. Reference was acidified H 2 O, ph=3.4. Table S4. Variable temperature reduced transverse and longitudinal 17 O relaxation rates of Gd 2 L 1 in the presence of 1M Ca 2+, c(gd 3+ )=45.6 mm, ph=7, P m = at T. Reference was acidified 1M CaCl 2. Table S5. Relaxometric Ca 2+ titration of Gd 2 L 1 at 25 C, ph neutral and T. Table S6. Relaxometric Ca 2+ titration of Gd 2 L 2 at 25 C, ph neutral and T. Table S7. Relaxometric Mg 2+ titration of Gd 2 L 1 at 25 C, ph neutral and T. Table S8. Luminescence lifetimes measurements on Eu 2 L 1 at 25 C, ph neutral, and the calculated hydration numbers q. Figure S1. Variable Ca 2+ concentration UV-Vis studies on Eu 2 L 1 at 50 C. Figure S2. Variable Ca 2+ concentration UV-Vis studies on Eu 2 L 1 at 37 C. Figure S3. Variable Ca 2+ concentration UV-Vis studies on Eu 2 L 1 at 25 C. Figure S4. Variable Ca 2+ concentration UV-Vis studies on Eu 2 L 1 at 15.5 C. Table S9. Fitted parameters of Gd 2 L 1 in the absence of Ca 2+. The underlined parameters were fixed during the fitting. Table S10. Fitted parameters of Gd 2 L 1 in the presence of 1M Ca 2+. The underlined parameters were fixed during the fitting. Figure S5. IR spectrum of the ligand L 1. Figure S6. IR spectrum of Gd 2 L 1 complex. Figure S7. 1 H NMR spectrum of the compound 6a. Figure S8. 13 C NMR spectrum of the compound 6a. Figure S9. 1 H NMR spectrum of the ligand L 1. Figure S C NMR spectrum of the ligand L 1. Figure S11. 1 H NMR spectrum of the ligand L 2. Figure S C NMR spectrum of the ligand L 2. Figure S13. 1 H- 1 H COSY spectrum of the compound 6a. Figure S14. 1 H- 13 C HSQC spectrum of the compound 6a.
2 Equations. 17 O NMR relaxation: From the measured 17 O NMR relaxation rates of the paramagnetic solutions, 1/T 1 and 1/T 2, and of the acidified water reference, 1/T 1 and 1/T 2, one can calculate the reduced relaxation rates, 1/T 1r, 1/T 2r (Eq. [1] and [2]), where 1/T 1m, 1/T 2m are the relaxation rates of the bound water and Δω m is the chemical shift difference between bound and bulk water, τ m is the mean residence time or the inverse of the water exchange rate k ex and P m is the mole fraction of the bound water. 1, = T1r P m T1 T = + [1] 1 T1m + τ m T1 OS T2m + τmt2m +Δωm 1 = T 2 2r P m T2 T = + [2] 2 τ m τ + T +Δω T2OS ( 2 ) m m m The terms 1/T 1OS and 1/T 2OS describe relaxation contributions from water molecules not directly bound to the paramagnetic centre. In previous studies it has been shown that 17 O outer sphere relaxation terms due to water molecules freely diffusing on the surface of Gd-polyaminocarboxylate complexes are negligible. For complexes with electronegative groups relaxation terms due to 2 nd sphere water molecules can however be important for longitudinal relaxation 1/T 1r and have therefore to be included = + = + [3] 1st 2nd 2nd T1r T1r T1r T1 m + τ m T1r T2m + τ m T2m +Δωm = = [4] 1st T 2 2r T2r τ m τ + T +Δω ( 2 ) m m m First sphere contribution to 17 O relaxation: The 17 O longitudinal relaxation rates in Gd(III) solutions are the sum of the contributions of the dipole-dipole and quadrupolar (in the approximation developed by Halle for non-extreme narrowing conditions) mechanisms as expressed by Eq. [6]-[7], where γ S is the electron and γ I is the nuclear gyromagnetic ratio (γ S = rad s -1 T -1, γ I = rad s -1 T -1 ), r GdO is the effective distance between the electron charge and the 17 O nucleus, I is the nuclear spin (5/2 for 17 O), χ is the quadrupolar coupling constant and η is an asymmetry parameter: = + [5] T T T 1m 1dd 1q μ 0 h I S = γ γ S 6 ( S 1) 3 J ( ωi; τd1) 7 J ( ωs; τd2) ; J ( ω; τ) τ 2 1dd 15 4π + + = r GdO 1 + T ( ωτ ) τ = d1 τ + m T + 1e τro π 2I η = χ J 2 ( ωi; τro) J ( ωi; τro) ; Jn ( ω; τ) = τ T1 q 10 I ( 2I 1) nωτ ( ) In the transverse relaxation the scalar contribution, 1/T 2sc, is dominating, Eq. [8]. 1/τ s1 is the sum of the exchange rate constant and the electron spin relaxation rate S( S + 1) = τ [8] S1 T2m T2SC 3 h = + [9] τ τ T S1 m 1e Second sphere contribution to 17 O relaxation: [6] [7]
3 2nd 2nd 1 q 1 q 1 1 = 2nd 1st 2nd 1st + 2nd 2nd T1 q T1m q T1dd T 1q 1 3τ =C + 2nd,O 2nd,O 2nd,O d1 d2 2nd dd 2 2 T1dd 1+ ωτ 1+ ωτ 7τ 2nd,O 2nd,O ( I d1 ) ( S d2 ) nd,O 2 μ 17 0 h γ γ O S C dd = S S+1 2nd π r ( GdO ) ( ) 1 3π 2I τ 0.8τ ( ) T I 2I 1 1+ ωτ 1+ 2ωτ 2 2nd,O 2nd,O 2 2 = χ 1+η 3 + 2nd q 10 ( ) = + τ τ τ τ O,2nd O O g l l τ 2nd =k 2nd,O ex + + O,2nd di τ T ie 2nd,O 2nd,O ( I ) ( I ) [10] [11] [12] [13] [14] 1 H NMRD: obs The measured longitudinal proton relaxation rate, R 1 is the sum of a paramagnetic and a diamagnetic contribution as expressed in Eq. [15], where r 1 is the proton relaxivity: obs d p d 3 R1 = R1 + R1 = R1 + r1 Gd + [15] The relaxivity is here given by the sum of inner sphere, second sphere and outer sphere contributions: r = r + r + r [16] 1 1is 1,2nd 1os Inner sphere 1 H relaxation: The inner sphere term is given in Eq. [17], where q 1st is the number of inner sphere water molecules. 3 1st 1 q 1 r1 is = [17] H T1 m + τ m The longitudinal relaxation rate of inner sphere protons, 1/T H 1m is expressed by Eq. [18], where r GdH is the effective distance between the electron charge and the 1 H nucleus, ω I is the proton resonance frequency and ω S is the Larmor frequency of the Gd(III) electron spin μ0 h γ Iγ S = 6 ( 1) 3 ( I; d1) 7 ( S; H S S + J ω τ + J ω τd2) T1 m 15 4π r [18] GdH 2 2 S τ ( 1 S ) τ dig di J ( ωτ ; di ) = + ; i = 1,2 [19] ωτdig 1+ ωτ di = + + ; H τ τ τ T τ = τ + τ + T ; i = 1,2 [20] dig m g ie = + H H τ τg τl dig m ie The spectral density functions are given by Eq. [19]. Second sphere 1 H relaxation: [21]
4 2nd 2nd 2nd 1 q 1 1 q 1 1 T 2nd,H 2nd,H 1dd + τ 2nd m T 1dd r = τ 2nd,H 2nd,H 2nd,O d1 d2 =C 2nd,H dd T1dd 1+ ωτ 1+ ωτ 7τ 2nd,H 2nd,H ( I d1 ) ( S d2 ) nd,H 2 μ 1 0 h γ γ H S C dd = S S+1 2nd π r ( GdO ) =k + + 2nd 2nd,H ex H τdi τ Tie = + H H τ τg τl ( ) [22] [23] [24] [25] [26] Outer sphere 1 H relaxation: The outer-sphere contribution can be described by Eq. [27] where N is the vogadro constant, and J os is its associated spectral density function. 4, N π μ0 h γ Sγ I 1os = + os I 1e + os S 2e 405 4π agdh DGdH ( 1) 3 ( ω ; ) 7 ( ω ; ) r S S J T J T 1/2 1 τ GdH 1+ iωτgdh + 4 Tje Jos ( ω, Tje ) = Re ; j = 1,2 [28] 1/2 3/2 τ GdH 4 τ GdH 1 τ GdH 1+ iωτgdh + + iωτgdh + + iωτgdh + T je 9 T je 9 T je 2 agdh τ GdH = [29] DGdH a GdH is the distance of closes approach and D GdH is the diffusion coefficient for the diffusion of a water proton relative to the Gd(III) complex. Electron spin relaxation: The longitudinal and transverse electronic relaxation rates, 1/T 1e and 1/T 2e are described by Solomon- Bloembergen-Morgan theory modified by Powell (Eqs. [30]-[31]), where τ V is the correlation time for the modulation of the zero-field-splitting interaction. ZFS = Δ τ v { 4S( S + 1) 3} [30] T1 e ωsτv 1+ 4ωSτv ZFS =Δ τ v [31] T2 e ωSτv ωSτv [27] Temperature dependences of water exchange rates and correlation times: The exchange rates are supposed to follow the Eyring equation. In Eq. [32] ΔS and ΔH are the entropy and enthalpy of activation for the water exchange process, and k ex 298 is the exchange rate at K. In Eq. [33] ΔH 2nd is the enthalpy of activation for the second sphere water exchange process and k ex 2nd,298 is the corresponding exchange rate at 298 K.
5 k k ex kt B ΔS ΔH kex T ΔH 1 1 = = exp = exp τ m h R RT R T k ΔH T T 2 nd,298 2nd 2nd ex 1 1 ex = exp ll correlation times and the diffusion constant are supposed to obey an rrhenius law: 298 E 1 1 exp a τ = τ R T EGdH 1 1 DGdH = DGdH exp R T [32] [33] [34] [35]
6 Table S1. Proton relaxivities (r 1 / mm -1 s -1 ) of Gd 2 L 1 in the absence of Ca 2+, c(gd 3+ )=5.1 mm, ph=6.25, at 25 and 37 C. ν ( 1 H)/MHz 15.5 C 25 C 37 C
7 Table S2. Proton relaxivities (r 1 / mm -1 s -1 ) of Gd 2 L 1 in the presence of Ca 2+, c(gd 3+ )=3.2 mm, c(ca 2+ )=4.4 mm, ph=7, at 15.5, 25 and 37 C. ν ( 1 H)/MHz 15.5 C 25 C
8 Table S3. Variable temperature reduced transverse and longitudinal 17 O relaxation rates of Gd 2 L 1 in the absence of Ca 2+, c(gd 3+ )=51.8 mm, ph=7, P m = at T. Reference was acidified H 2 O, ph=3.4. t / C T / K 1000/T / K -1 P m T 1 (Gd)/s T 1 (ref)/s T 2 (Gd)/s T 2 (ref)/s ln(1/t 1r ) ln(1/t 2r ) E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E Table S4. Variable temperature reduced transverse and longitudinal 17 O relaxation rates of Gd 2 L 1 in the presence of 1M Ca 2+, c(gd 3+ )=45.6 mm, ph=7, P m = at T. Reference was acidified 1M CaCl 2. t / C T / K 1000/T / K -1 P m T 1 (Gd)/s T 1 (ref)/s T 2 (Gd)/s T 2 (ref)/s ln(1/t 1r ) ln(1/t 2r ) E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E E
9 Table S5. Relaxometric Ca 2+ titration of Gd 2 L 1 at 25 C, ph 7 and T. c(gd 3+ ) / mm c(ca 2+ ) / mm c(complex) / mm n(ca 2+ ):n(complex) T 1 / ms r 1 / mmol -1 s Table S6. Relaxometric Ca 2+ titration of Gd 2 L 2 at 25 C, ph 7 and T. c(gd 3+ ) / mm c(ca 2+ ) / mm c(complex) / mm n(ca 2+ ):n(complex) T 1 / r 1 / mmol -1 s -1 ms
10 Table S7. Relaxometric Mg 2+ titration of Gd 2 L 1 at 25 C, ph 7 and T. c(gd 3+ ) / mm c(mg 2+ ) / mm c(ca 2+ ) / mm c(complex) / mm n(mg 2+ ):n(complex) n(ca 2+ ):n(complex) r 1 / mmol -1 s
11 Table S8. Luminescence lifetimes measurements on Eu 2 L 1 at 25 C, ph neutral, and the calculated hydration numbers q. n(ca 2+ ):n(complex) τ H2O / ms τ D2O / ms q
12 Figure S1. Variable Ca 2+ concentration UV-Vis studies on Eu 2 L 1 at 50 C. 1,4 equiv. Ca(II) 0,15 0,13 0,11 0,09 0,07 0 equiv. Ca(II) 0,84 0,82 0,8 0,78 0,05 0,03 0,01-0,01-0,03-0,05 10 equiv. Ca(II) 0,76 0,74 0,72 0,7 50 equiv. Ca(II) 0,12 0,1 0,08 0,06 0,04 0,0005 0,0004 0,0003 0,0002 0, ,02-0, ,0002 Figure S2. Variable Ca 2+ concentration UV-Vis studies on Eu 2 L 1 at 37 C. 0 equiv. Ca(II) 1,4 equiv. Ca(II) 0,15 0,84 0,13 0,11 0,09 0,82 0,8 0,07 0,05 0,03 0,01-0,01-0,03-0,05 0,78 0,76 0,74 0,72 0,7 10 equiv. Ca(II) 50 equiv. Ca(II) 0,2 0,19 0,18 0,17 0,0012 0,001 0,0008 0,16 0,15 0,14 0,13 0,12 0,11 0,1 0,0006 0,0004 0, ,0002
13 Figure S3. Variable Ca 2+ concentration UV-Vis studies on Eu 2 L 1 at 25 C. 0 equiv. Ca(II) 1,4 equiv. Ca(II) 0,2 0,1 0,1 0,1 0,1 0,1 0,0 0,0 0,0 0,0 0,15 0,13 0,11 0,09 0,07 0,05 0,03 0,01-0,01-0,03-0,05 10 equiv. Ca(II) 50 equiv. Ca(II) 0,18 0, ,16 0,0002 0, ,14 0,0001 0,12 0, ,1 0-0, ,08-0,0001 0,06-0,00015 Figure S4. Variable Ca 2+ concentration UV-Vis studies on Eu 2 L 1 at 15.5 C. 1,4 equiv.ca(ii) 10 equiv. Ca(II) 0,34 0,02 0,32 0 0,3-0,02 0,28 0,26-0,04 0,24-0,06 0,22-0,08 0,2-0,1
14 Table S9. Fitted parameters of Gd 2 L 1 in the absence of Ca 2+. The underlined parameters were fixed during the fitting. Parameter Gd 2 L 1 k 298 ex [10 6 s -1 ] 2.4±0.2 ΔH [kj mol -1 ] 43.6±3.3 ΔS [J mol -1 K -1 ] /ħ [10 6 rad s -1 ] -3.8 τ 298 RO [ps] 349±47 Ε R [kj mol -1 ] 24±1 τ 298 V [ps] 20.6±2.7 Ε V [kj mol -1 ] 1 Δ 2 [10 20 s -2 ] 0.46±0.10 D 298 GdH [10-10 m 2 s -1 ] 25±3 Ε DGdH [kj mol -1 ] 30±2 δg 2 L [10-1 ] 2.7±0.7 τ RH / τ RO 0.76±0.12 r GdO [Å] 2.5 r GdH [Å] 3.1 r GdHouter [Å] 3.6 χ(1+η 2 /3) 1/2 [MHz] 7.58 q 0.4 q 2nd τ M 2nd [ps] 50 ΔH 298 2nd [kj mol -1 ] 35 r 2nd GdH [Å] 3.5 r 2nd GdO [Å] 4.1
15 Table S10. Fitted parameters of Gd 2 L 1 in the presence of 1M Ca 2+. The underlined parameters were fixed during the fitting. Parameter Gd 2 L 1 + Ca 2+ k 298 ex [10 6 s -1 ] 7.5±1.6 ΔH [kj mol -1 ] 43.6 ΔS [J mol -1 K -1 ] /ħ [10 6 rad s -1 ] -3.8 τ 298 RO [ps] 1152±243 Ε R [kj mol -1 ] 21±6 τ 298 V [ps] 0.13±0.02 Ε V [kj mol -1 ] 1 Δ 2 [10 20 s -2 ] 0.50±0.05 δg 2 L [10-2 ] 2.1 r GdO [Å] 2.5 r GdH [Å] 3.1 r GdHouter [Å] 3.5 χ(1+η 2 /3) 1/2 [MHz] 7.58 q 0.7 q 2nd τ M 2nd [ps] 50 ΔH 298 2nd [kj mol -1 ] 35 r 2nd GdH [Å] 3.5 r 2nd GdO [Å] 4.1
16 Figure S5. IR spectrum of the ligand L 1.
17 Figure S6. IR spectrum of Gd 2 L 1 complex.
18 Figure S7. 1 H NMR spectrum of the compound 6a.
19 Figure S8. 13 C NMR spectrum of the compound 6a.
20 Figure S9. 1 H NMR spectrum of the ligand L 1.
21 Figure S C NMR spectrum of the ligand L 1.
22 Figure S11. 1 H NMR spectrum of the ligand L 2.
23 Figure S C NMR spectrum of the ligand L 2.
24 Figure S13. 1 H- 1 H COSY spectrum of the compound 6a.
25 Figure S14. 1 H- 13 C HSQC spectrum of the compound 6a. References 1 T. J. Swift, R. E. Connick, J. Chem. Phys., 1962, 37, J. R. Zimmermann, W. E. Brittin, J. Phys. Chem., 1957, 61, Z. Luz, S. Meiboom, J. Chem. Phys., 1964, 40, J. H. Freed, J. Chem. Phys., 1978, 68, S. H. Koenig, R. D. Brown III, Prog. Nucl. Magn. Reson. Spectrosc., 1991, 22, 487.
Phosphinic derivative of DTPA conjugated to a G5 PAMAM dendrimer: an. Supplementary information
 Phosphinic derivative of DTPA conjugated to a G5 PAMAM dendrier: an 17 O and 1 H relaxation study of its Gd(III) coplex Petra Lebdušková, ab Angélique Sour, b Lothar Hel,* b Éva Tóth, bc Jan Kotek, a Ivan
Phosphinic derivative of DTPA conjugated to a G5 PAMAM dendrier: an 17 O and 1 H relaxation study of its Gd(III) coplex Petra Lebdušková, ab Angélique Sour, b Lothar Hel,* b Éva Tóth, bc Jan Kotek, a Ivan
DuPont Suva 95 Refrigerant
 Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Mean bond enthalpy Standard enthalpy of formation Bond N H N N N N H O O O
 Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
DuPont Suva 95 Refrigerant
 Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
DuPont Suva. DuPont. Thermodynamic Properties of. Refrigerant (R-410A) Technical Information. refrigerants T-410A ENG
 Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
of the methanol-dimethylamine complex
 Electronic Supplementary Information for: Fundamental and overtone virational spectroscopy, enthalpy of hydrogen ond formation and equilirium constant determination of the methanol-dimethylamine complex
Electronic Supplementary Information for: Fundamental and overtone virational spectroscopy, enthalpy of hydrogen ond formation and equilirium constant determination of the methanol-dimethylamine complex
Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]
![Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)] Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]](/thumbs/89/98928029.jpg) d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
Supporting Information
 Chloromethylhalicyclamine B, a Marine-Derived Protein Kinase CK1δ/ε Inhibitor Germana Esposito, Marie-Lise Bourguet-Kondracki, * Linh H. Mai, Arlette Longeon, Roberta Teta, Laurent Meijer, Rob Van Soest,
Chloromethylhalicyclamine B, a Marine-Derived Protein Kinase CK1δ/ε Inhibitor Germana Esposito, Marie-Lise Bourguet-Kondracki, * Linh H. Mai, Arlette Longeon, Roberta Teta, Laurent Meijer, Rob Van Soest,
CHAPTER 25 SOLVING EQUATIONS BY ITERATIVE METHODS
 CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
Section 8.3 Trigonometric Equations
 99 Section 8. Trigonometric Equations Objective 1: Solve Equations Involving One Trigonometric Function. In this section and the next, we will exple how to solving equations involving trigonometric functions.
99 Section 8. Trigonometric Equations Objective 1: Solve Equations Involving One Trigonometric Function. In this section and the next, we will exple how to solving equations involving trigonometric functions.
6.1. Dirac Equation. Hamiltonian. Dirac Eq.
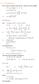 6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
w o = R 1 p. (1) R = p =. = 1
 Πανεπιστήµιο Κρήτης - Τµήµα Επιστήµης Υπολογιστών ΗΥ-570: Στατιστική Επεξεργασία Σήµατος 205 ιδάσκων : Α. Μουχτάρης Τριτη Σειρά Ασκήσεων Λύσεις Ασκηση 3. 5.2 (a) From the Wiener-Hopf equation we have:
Πανεπιστήµιο Κρήτης - Τµήµα Επιστήµης Υπολογιστών ΗΥ-570: Στατιστική Επεξεργασία Σήµατος 205 ιδάσκων : Α. Μουχτάρης Τριτη Σειρά Ασκήσεων Λύσεις Ασκηση 3. 5.2 (a) From the Wiener-Hopf equation we have:
Higher Derivative Gravity Theories
 Higher Derivative Gravity Theories Black Holes in AdS space-times James Mashiyane Supervisor: Prof Kevin Goldstein University of the Witwatersrand Second Mandelstam, 20 January 2018 James Mashiyane WITS)
Higher Derivative Gravity Theories Black Holes in AdS space-times James Mashiyane Supervisor: Prof Kevin Goldstein University of the Witwatersrand Second Mandelstam, 20 January 2018 James Mashiyane WITS)
Si + Al Mg Fe + Mn +Ni Ca rim Ca p.f.u
 .6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
.6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
DESIGN OF MACHINERY SOLUTION MANUAL h in h 4 0.
 DESIGN OF MACHINERY SOLUTION MANUAL -7-1! PROBLEM -7 Statement: Design a double-dwell cam to move a follower from to 25 6, dwell for 12, fall 25 and dwell for the remader The total cycle must take 4 sec
DESIGN OF MACHINERY SOLUTION MANUAL -7-1! PROBLEM -7 Statement: Design a double-dwell cam to move a follower from to 25 6, dwell for 12, fall 25 and dwell for the remader The total cycle must take 4 sec
LP N to BD* C-C = BD C-C to BD* O-H = LP* C to LP* B =5.
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2016 MS No.: CP-ART-03-2016-002134.R1 Optical Response and Gas Sequestration Properties
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2016 MS No.: CP-ART-03-2016-002134.R1 Optical Response and Gas Sequestration Properties
Supplementary Materials for. Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides
 Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Copper(II) complexes of salicylaldehydes and 2 hydroxyphenones: Synthesis, Structure,
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Copper(II) complexes of salicylaldehydes and 2 hydroxyphenones: Synthesis, Structure, Thermal
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Copper(II) complexes of salicylaldehydes and 2 hydroxyphenones: Synthesis, Structure, Thermal
Supporting Information
 Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
An experimental and theoretical study of the gas phase kinetics of atomic chlorine reactions with CH 3 NH 2, (CH 3 ) 2 NH, and (CH 3 ) 3 N
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Heronamides A C, new polyketide macrolactams from an Australian marine-derived Streptomyces sp. A biosynthetic case for synchronized tandem electrocyclization. Ritesh Raju, Andrew
SUPPORTING INFORMATION Heronamides A C, new polyketide macrolactams from an Australian marine-derived Streptomyces sp. A biosynthetic case for synchronized tandem electrocyclization. Ritesh Raju, Andrew
Engineering Tunable Single and Dual Optical. Emission from Ru(II)-Polypyridyl Complexes. Through Excited State Design
 Engineering Tunable Single and Dual Optical Emission from Ru(II)-Polypyridyl Complexes Through Excited State Design Supplementary Information Julia Romanova 1, Yousif Sadik 1, M. R. Ranga Prabhath 1,,
Engineering Tunable Single and Dual Optical Emission from Ru(II)-Polypyridyl Complexes Through Excited State Design Supplementary Information Julia Romanova 1, Yousif Sadik 1, M. R. Ranga Prabhath 1,,
Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes
 Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes Nathalia B. D. Lima [a], José Diogo L. Dutra [a,b], Simone M. C. Gonçalves [a], Ricardo O.
Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes Nathalia B. D. Lima [a], José Diogo L. Dutra [a,b], Simone M. C. Gonçalves [a], Ricardo O.
MATH423 String Theory Solutions 4. = 0 τ = f(s). (1) dτ ds = dxµ dτ f (s) (2) dτ 2 [f (s)] 2 + dxµ. dτ f (s) (3)
![MATH423 String Theory Solutions 4. = 0 τ = f(s). (1) dτ ds = dxµ dτ f (s) (2) dτ 2 [f (s)] 2 + dxµ. dτ f (s) (3) MATH423 String Theory Solutions 4. = 0 τ = f(s). (1) dτ ds = dxµ dτ f (s) (2) dτ 2 [f (s)] 2 + dxµ. dτ f (s) (3)](/thumbs/81/84712331.jpg) 1. MATH43 String Theory Solutions 4 x = 0 τ = fs). 1) = = f s) ) x = x [f s)] + f s) 3) equation of motion is x = 0 if an only if f s) = 0 i.e. fs) = As + B with A, B constants. i.e. allowe reparametrisations
1. MATH43 String Theory Solutions 4 x = 0 τ = fs). 1) = = f s) ) x = x [f s)] + f s) 3) equation of motion is x = 0 if an only if f s) = 0 i.e. fs) = As + B with A, B constants. i.e. allowe reparametrisations
Jesse Maassen and Mark Lundstrom Purdue University November 25, 2013
 Notes on Average Scattering imes and Hall Factors Jesse Maassen and Mar Lundstrom Purdue University November 5, 13 I. Introduction 1 II. Solution of the BE 1 III. Exercises: Woring out average scattering
Notes on Average Scattering imes and Hall Factors Jesse Maassen and Mar Lundstrom Purdue University November 5, 13 I. Introduction 1 II. Solution of the BE 1 III. Exercises: Woring out average scattering
Homework 8 Model Solution Section
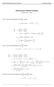 MATH 004 Homework Solution Homework 8 Model Solution Section 14.5 14.6. 14.5. Use the Chain Rule to find dz where z cosx + 4y), x 5t 4, y 1 t. dz dx + dy y sinx + 4y)0t + 4) sinx + 4y) 1t ) 0t + 4t ) sinx
MATH 004 Homework Solution Homework 8 Model Solution Section 14.5 14.6. 14.5. Use the Chain Rule to find dz where z cosx + 4y), x 5t 4, y 1 t. dz dx + dy y sinx + 4y)0t + 4) sinx + 4y) 1t ) 0t + 4t ) sinx
Phys460.nb Solution for the t-dependent Schrodinger s equation How did we find the solution? (not required)
 Phys460.nb 81 ψ n (t) is still the (same) eigenstate of H But for tdependent H. The answer is NO. 5.5.5. Solution for the tdependent Schrodinger s equation If we assume that at time t 0, the electron starts
Phys460.nb 81 ψ n (t) is still the (same) eigenstate of H But for tdependent H. The answer is NO. 5.5.5. Solution for the tdependent Schrodinger s equation If we assume that at time t 0, the electron starts
difluoroboranyls derived from amides carrying donor group Supporting Information
 The influence of the π-conjugated spacer on photophysical properties of difluoroboranyls derived from amides carrying donor group Supporting Information Anna Maria Grabarz a Adèle D. Laurent b, Beata Jędrzejewska
The influence of the π-conjugated spacer on photophysical properties of difluoroboranyls derived from amides carrying donor group Supporting Information Anna Maria Grabarz a Adèle D. Laurent b, Beata Jędrzejewska
9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer catalysts
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer
Derivation of Optical-Bloch Equations
 Appendix C Derivation of Optical-Bloch Equations In this appendix the optical-bloch equations that give the populations and coherences for an idealized three-level Λ system, Fig. 3. on page 47, will be
Appendix C Derivation of Optical-Bloch Equations In this appendix the optical-bloch equations that give the populations and coherences for an idealized three-level Λ system, Fig. 3. on page 47, will be
Electronic Supplementary Information (ESI)
 Electronic Supplementary Information (ESI) Lanthanide metal-organic frameworks constructed by asymmetric 2-nitro-biphenyl-4,4 -dicarboxylate ligand: syntheses, structures, luminescence and magnetic investigations
Electronic Supplementary Information (ESI) Lanthanide metal-organic frameworks constructed by asymmetric 2-nitro-biphenyl-4,4 -dicarboxylate ligand: syntheses, structures, luminescence and magnetic investigations
Supplementary Information
 9.4 6.60. 8.6 8.8 9.29 20.04 29.6 0.07 6. 40.92 4.64 06.0 6.29 6.0-0.00 Supplementary Information Conformational Analysis, Experimental and GIA-DFT C NMR Chemical Shift Calculation on 2 -Hydroxy-,4,-trimethoxy-chalcone
9.4 6.60. 8.6 8.8 9.29 20.04 29.6 0.07 6. 40.92 4.64 06.0 6.29 6.0-0.00 Supplementary Information Conformational Analysis, Experimental and GIA-DFT C NMR Chemical Shift Calculation on 2 -Hydroxy-,4,-trimethoxy-chalcone
[1] P Q. Fig. 3.1
![[1] P Q. Fig. 3.1 [1] P Q. Fig. 3.1](/thumbs/79/80362156.jpg) 1 (a) Define resistance....... [1] (b) The smallest conductor within a computer processing chip can be represented as a rectangular block that is one atom high, four atoms wide and twenty atoms long. One
1 (a) Define resistance....... [1] (b) The smallest conductor within a computer processing chip can be represented as a rectangular block that is one atom high, four atoms wide and twenty atoms long. One
Nitric oxide (NO) reactivity studies on mononuclear Iron(II) complexes supported by a tetradentate Schiff base Ligand
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Nitric oxide (NO) reactivity studies on mononuclear Iron(II) complexes supported by a tetradentate
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Nitric oxide (NO) reactivity studies on mononuclear Iron(II) complexes supported by a tetradentate
Approximation of distance between locations on earth given by latitude and longitude
 Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
HOMEWORK 4 = G. In order to plot the stress versus the stretch we define a normalized stretch:
 HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
the total number of electrons passing through the lamp.
 1. A 12 V 36 W lamp is lit to normal brightness using a 12 V car battery of negligible internal resistance. The lamp is switched on for one hour (3600 s). For the time of 1 hour, calculate (i) the energy
1. A 12 V 36 W lamp is lit to normal brightness using a 12 V car battery of negligible internal resistance. The lamp is switched on for one hour (3600 s). For the time of 1 hour, calculate (i) the energy
Matrices and Determinants
 Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
(1) Describe the process by which mercury atoms become excited in a fluorescent tube (3)
 Q1. (a) A fluorescent tube is filled with mercury vapour at low pressure. In order to emit electromagnetic radiation the mercury atoms must first be excited. (i) What is meant by an excited atom? (1) (ii)
Q1. (a) A fluorescent tube is filled with mercury vapour at low pressure. In order to emit electromagnetic radiation the mercury atoms must first be excited. (i) What is meant by an excited atom? (1) (ii)
Supporting Information
 Supporting Information On the Ambiguity of 1,3,2-Benzodiazaboroles as Donor/Acceptor unctionalities in Luminescent Molecules Lothar Weber *[a], Johannes Halama [a], Kenny Hanke [a], Lena Böhling [a], Andreas
Supporting Information On the Ambiguity of 1,3,2-Benzodiazaboroles as Donor/Acceptor unctionalities in Luminescent Molecules Lothar Weber *[a], Johannes Halama [a], Kenny Hanke [a], Lena Böhling [a], Andreas
Correction Table for an Alcoholometer Calibrated at 20 o C
 An alcoholometer is a device that measures the concentration of ethanol in a water-ethanol mixture (often in units of %abv percent alcohol by volume). The depth to which an alcoholometer sinks in a water-ethanol
An alcoholometer is a device that measures the concentration of ethanol in a water-ethanol mixture (often in units of %abv percent alcohol by volume). The depth to which an alcoholometer sinks in a water-ethanol
Supporting Information. Experimental section
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Appendix to On the stability of a compressible axisymmetric rotating flow in a pipe. By Z. Rusak & J. H. Lee
 Appendi to On the stability of a compressible aisymmetric rotating flow in a pipe By Z. Rusak & J. H. Lee Journal of Fluid Mechanics, vol. 5 4, pp. 5 4 This material has not been copy-edited or typeset
Appendi to On the stability of a compressible aisymmetric rotating flow in a pipe By Z. Rusak & J. H. Lee Journal of Fluid Mechanics, vol. 5 4, pp. 5 4 This material has not been copy-edited or typeset
Solutions to Exercise Sheet 5
 Solutions to Eercise Sheet 5 jacques@ucsd.edu. Let X and Y be random variables with joint pdf f(, y) = 3y( + y) where and y. Determine each of the following probabilities. Solutions. a. P (X ). b. P (X
Solutions to Eercise Sheet 5 jacques@ucsd.edu. Let X and Y be random variables with joint pdf f(, y) = 3y( + y) where and y. Determine each of the following probabilities. Solutions. a. P (X ). b. P (X
Supporting Information One-Pot Approach to Chiral Chromenes via Enantioselective Organocatalytic Domino Oxa-Michael-Aldol Reaction
 Supporting Information ne-pot Approach to Chiral Chromenes via Enantioselective rganocatalytic Domino xa-michael-aldol Reaction Hao Li, Jian Wang, Timiyin E-Nunu, Liansuo Zu, Wei Jiang, Shaohua Wei, *
Supporting Information ne-pot Approach to Chiral Chromenes via Enantioselective rganocatalytic Domino xa-michael-aldol Reaction Hao Li, Jian Wang, Timiyin E-Nunu, Liansuo Zu, Wei Jiang, Shaohua Wei, *
Electronic Supplementary Information:
 Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Figure 1 T / K Explain, in terms of molecules, why the first part of the graph in Figure 1 is a line that slopes up from the origin.
 Q1.(a) Figure 1 shows how the entropy of a molecular substance X varies with temperature. Figure 1 T / K (i) Explain, in terms of molecules, why the entropy is zero when the temperature is zero Kelvin.
Q1.(a) Figure 1 shows how the entropy of a molecular substance X varies with temperature. Figure 1 T / K (i) Explain, in terms of molecules, why the entropy is zero when the temperature is zero Kelvin.
A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
Reaction of a Platinum Electrode for the Measurement of Redox Potential of Paddy Soil
 J. Jpn. Soc. Soil Phys. No. +*0, p.- +*,**1 Eh * ** Reaction of a Platinum Electrode for the Measurement of Redox Potential of Paddy Soil Daisuke MURAKAMI* and Tatsuaki KASUBUCHI** * The United Graduate
J. Jpn. Soc. Soil Phys. No. +*0, p.- +*,**1 Eh * ** Reaction of a Platinum Electrode for the Measurement of Redox Potential of Paddy Soil Daisuke MURAKAMI* and Tatsuaki KASUBUCHI** * The United Graduate
Enantioselective Organocatalytic Michael Addition of Isorhodanines. to α, β-unsaturated Aldehydes
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Supporting Information. Palladium Complexes with Bulky Diphosphine. Synthesis of (Bio-) Adipic Acid from Pentenoic. Acid Mixtures.
 Supporting Information Palladium Complexes with Bulky Diphosphine Ligands as Highly Selective Catalysts for the Synthesis of (Bio-) Adipic Acid from Pentenoic Acid Mixtures. Choon Heng Low, James D. Nobbs,*
Supporting Information Palladium Complexes with Bulky Diphosphine Ligands as Highly Selective Catalysts for the Synthesis of (Bio-) Adipic Acid from Pentenoic Acid Mixtures. Choon Heng Low, James D. Nobbs,*
3.4 SUM AND DIFFERENCE FORMULAS. NOTE: cos(α+β) cos α + cos β cos(α-β) cos α -cos β
 3.4 SUM AND DIFFERENCE FORMULAS Page Theorem cos(αβ cos α cos β -sin α cos(α-β cos α cos β sin α NOTE: cos(αβ cos α cos β cos(α-β cos α -cos β Proof of cos(α-β cos α cos β sin α Let s use a unit circle
3.4 SUM AND DIFFERENCE FORMULAS Page Theorem cos(αβ cos α cos β -sin α cos(α-β cos α cos β sin α NOTE: cos(αβ cos α cos β cos(α-β cos α -cos β Proof of cos(α-β cos α cos β sin α Let s use a unit circle
D-Glucosamine-derived copper catalyst for Ullmann-type C- N coupling reaction: theoretical and experimental study
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 D-Glucosamine-derived copper catalyst for Ullmann-type C- N coupling reaction: theoretical
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 D-Glucosamine-derived copper catalyst for Ullmann-type C- N coupling reaction: theoretical
Supporting Information
 Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany An Unusual Supramolecular Building Block: the Mixed Group 15/16 Element Ligand Complex [(Cp*Mo) 2 (µ,η 3 - P 3 )(µ,η 2 -PS)] Laurence J. Gregoriades,
Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany An Unusual Supramolecular Building Block: the Mixed Group 15/16 Element Ligand Complex [(Cp*Mo) 2 (µ,η 3 - P 3 )(µ,η 2 -PS)] Laurence J. Gregoriades,
SUPPORTING INFORMATION
 SUPPRTING INFRMATIN Per(-guanidino--deoxy)cyclodextrins: Synthesis, characterisation and binding behaviour toward selected small molecules and DNA. Nikolaos Mourtzis, a Kyriaki Eliadou, a Chrysie Aggelidou,
SUPPRTING INFRMATIN Per(-guanidino--deoxy)cyclodextrins: Synthesis, characterisation and binding behaviour toward selected small molecules and DNA. Nikolaos Mourtzis, a Kyriaki Eliadou, a Chrysie Aggelidou,
Divergent synthesis of various iminocyclitols from D-ribose
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 205 Divergent synthesis of various iminocyclitols from D-ribose Ramu Petakamsetty,
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 205 Divergent synthesis of various iminocyclitols from D-ribose Ramu Petakamsetty,
Srednicki Chapter 55
 Srednicki Chapter 55 QFT Problems & Solutions A. George August 3, 03 Srednicki 55.. Use equations 55.3-55.0 and A i, A j ] = Π i, Π j ] = 0 (at equal times) to verify equations 55.-55.3. This is our third
Srednicki Chapter 55 QFT Problems & Solutions A. George August 3, 03 Srednicki 55.. Use equations 55.3-55.0 and A i, A j ] = Π i, Π j ] = 0 (at equal times) to verify equations 55.-55.3. This is our third
Lecture 2: Dirac notation and a review of linear algebra Read Sakurai chapter 1, Baym chatper 3
 Lecture 2: Dirac notation and a review of linear algebra Read Sakurai chapter 1, Baym chatper 3 1 State vector space and the dual space Space of wavefunctions The space of wavefunctions is the set of all
Lecture 2: Dirac notation and a review of linear algebra Read Sakurai chapter 1, Baym chatper 3 1 State vector space and the dual space Space of wavefunctions The space of wavefunctions is the set of all
Trigonometric Formula Sheet
 Trigonometric Formula Sheet Definition of the Trig Functions Right Triangle Definition Assume that: 0 < θ < or 0 < θ < 90 Unit Circle Definition Assume θ can be any angle. y x, y hypotenuse opposite θ
Trigonometric Formula Sheet Definition of the Trig Functions Right Triangle Definition Assume that: 0 < θ < or 0 < θ < 90 Unit Circle Definition Assume θ can be any angle. y x, y hypotenuse opposite θ
DERIVATION OF MILES EQUATION FOR AN APPLIED FORCE Revision C
 DERIVATION OF MILES EQUATION FOR AN APPLIED FORCE Revision C By Tom Irvine Email: tomirvine@aol.com August 6, 8 Introduction The obective is to derive a Miles equation which gives the overall response
DERIVATION OF MILES EQUATION FOR AN APPLIED FORCE Revision C By Tom Irvine Email: tomirvine@aol.com August 6, 8 Introduction The obective is to derive a Miles equation which gives the overall response
( ) 2 and compare to M.
 Problems and Solutions for Section 4.2 4.9 through 4.33) 4.9 Calculate the square root of the matrix 3!0 M!0 8 Hint: Let M / 2 a!b ; calculate M / 2!b c ) 2 and compare to M. Solution: Given: 3!0 M!0 8
Problems and Solutions for Section 4.2 4.9 through 4.33) 4.9 Calculate the square root of the matrix 3!0 M!0 8 Hint: Let M / 2 a!b ; calculate M / 2!b c ) 2 and compare to M. Solution: Given: 3!0 M!0 8
Fundamental Physical Constants Extensive Listing Relative std. Quantity Symbol Value Unit uncert. u r
 UNIVERSAL speed of light in vacuum c, c 0 299 792 458 m s 1 exact magnetic constant µ 0 4π 10 7 N A 2 = 12.566 370 614... 10 7 N A 2 exact electric constant 1/µ 0 c 2 ɛ 0 8.854 187 817... 10 12 F m 1 exact
UNIVERSAL speed of light in vacuum c, c 0 299 792 458 m s 1 exact magnetic constant µ 0 4π 10 7 N A 2 = 12.566 370 614... 10 7 N A 2 exact electric constant 1/µ 0 c 2 ɛ 0 8.854 187 817... 10 12 F m 1 exact
Potential Dividers. 46 minutes. 46 marks. Page 1 of 11
 Potential Dividers 46 minutes 46 marks Page 1 of 11 Q1. In the circuit shown in the figure below, the battery, of negligible internal resistance, has an emf of 30 V. The pd across the lamp is 6.0 V and
Potential Dividers 46 minutes 46 marks Page 1 of 11 Q1. In the circuit shown in the figure below, the battery, of negligible internal resistance, has an emf of 30 V. The pd across the lamp is 6.0 V and
Second Order RLC Filters
 ECEN 60 Circuits/Electronics Spring 007-0-07 P. Mathys Second Order RLC Filters RLC Lowpass Filter A passive RLC lowpass filter (LPF) circuit is shown in the following schematic. R L C v O (t) Using phasor
ECEN 60 Circuits/Electronics Spring 007-0-07 P. Mathys Second Order RLC Filters RLC Lowpass Filter A passive RLC lowpass filter (LPF) circuit is shown in the following schematic. R L C v O (t) Using phasor
2. Chemical Thermodynamics and Energetics - I
 . Chemical Thermodynamics and Energetics - I 1. Given : Initial Volume ( = 5L dm 3 Final Volume (V = 10L dm 3 ext = 304 cm of Hg Work done W = ext V ext = 304 cm of Hg = 304 atm [... 76cm of Hg = 1 atm]
. Chemical Thermodynamics and Energetics - I 1. Given : Initial Volume ( = 5L dm 3 Final Volume (V = 10L dm 3 ext = 304 cm of Hg Work done W = ext V ext = 304 cm of Hg = 304 atm [... 76cm of Hg = 1 atm]
Supporting Information. for. Single-molecule magnet behavior in 2,2 -bipyrimidinebridged
 Supporting Information for Single-molecule magnet behavior in 2,2 -bipyrimidinebridged dilanthanide complexes Wen Yu 1*, Frank Schramm 1, Eufemio Moreno Pineda 1, Yanhua Lan 2, Olaf Fuhr 1, Jinjie Chen
Supporting Information for Single-molecule magnet behavior in 2,2 -bipyrimidinebridged dilanthanide complexes Wen Yu 1*, Frank Schramm 1, Eufemio Moreno Pineda 1, Yanhua Lan 2, Olaf Fuhr 1, Jinjie Chen
Supplementary Information
 Electronic upplementary Material (EI) for Photochemical & Photobiological ciences. This journal is The Royal ociety of Chemistry and wner ocieties 214 upplementary Information elective and sensitive fluorescence-shift
Electronic upplementary Material (EI) for Photochemical & Photobiological ciences. This journal is The Royal ociety of Chemistry and wner ocieties 214 upplementary Information elective and sensitive fluorescence-shift
Patrycja Miszczyk, Dorota Wieczorek, Joanna Gałęzowska, Błażej Dziuk, Joanna Wietrzyk and Ewa Chmielewska. 1. Spectroscopic Data.
 ; doi:10.3390/molecules22020254 S1 of S23 Supplementary Materials: Reaction of 3-Amino-1,2,4-Triazole with Diethyl Phosphite and Triethyl Orthoformate: Acid-Base Properties and Antiosteoporotic Activities
; doi:10.3390/molecules22020254 S1 of S23 Supplementary Materials: Reaction of 3-Amino-1,2,4-Triazole with Diethyl Phosphite and Triethyl Orthoformate: Acid-Base Properties and Antiosteoporotic Activities
Fundamental Physical Constants Extensive Listing Relative std. Quantity Symbol Value Unit uncert. u r
 UNIVERSAL speed of light in vacuum c, c 0 299 792 458 m s 1 exact magnetic constant µ 0 4π 10 7 N A 2 = 12.566 370 614... 10 7 N A 2 exact electric constant 1/µ 0 c 2 ɛ 0 8.854 187 817... 10 12 F m 1 exact
UNIVERSAL speed of light in vacuum c, c 0 299 792 458 m s 1 exact magnetic constant µ 0 4π 10 7 N A 2 = 12.566 370 614... 10 7 N A 2 exact electric constant 1/µ 0 c 2 ɛ 0 8.854 187 817... 10 12 F m 1 exact
Fused Bis-Benzothiadiazoles as Electron Acceptors
 Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Example Sheet 3 Solutions
 Example Sheet 3 Solutions. i Regular Sturm-Liouville. ii Singular Sturm-Liouville mixed boundary conditions. iii Not Sturm-Liouville ODE is not in Sturm-Liouville form. iv Regular Sturm-Liouville note
Example Sheet 3 Solutions. i Regular Sturm-Liouville. ii Singular Sturm-Liouville mixed boundary conditions. iii Not Sturm-Liouville ODE is not in Sturm-Liouville form. iv Regular Sturm-Liouville note
Solutions to the Schrodinger equation atomic orbitals. Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz
 Solutions to the Schrodinger equation atomic orbitals Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz ybridization Valence Bond Approach to bonding sp 3 (Ψ 2 s + Ψ 2 px + Ψ 2 py + Ψ 2 pz) sp 2 (Ψ 2 s + Ψ 2 px + Ψ 2 py)
Solutions to the Schrodinger equation atomic orbitals Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz ybridization Valence Bond Approach to bonding sp 3 (Ψ 2 s + Ψ 2 px + Ψ 2 py + Ψ 2 pz) sp 2 (Ψ 2 s + Ψ 2 px + Ψ 2 py)
By R.L. Snyder (Revised March 24, 2005)
 Humidity Conversion By R.L. Snyder (Revised March 24, 2005) This Web page provides the equations used to make humidity conversions and tables o saturation vapor pressure. For a pd ile o this document,
Humidity Conversion By R.L. Snyder (Revised March 24, 2005) This Web page provides the equations used to make humidity conversions and tables o saturation vapor pressure. For a pd ile o this document,
Site-Selective Suzuki-Miyaura Cross-Coupling Reactions of 2,3,4,5-Tetrabromofuran
 1 Site-Selective Suzuki-Miyaura Cross-Coupling Reactions of 2,3,4,5-Tetrabromofuran Munawar Hussain, a Rasheed Ahmad Khera, a Nguyen Thai Hung, a Peter Langer* a,b a Institut für Chemie, Universität Rostock,
1 Site-Selective Suzuki-Miyaura Cross-Coupling Reactions of 2,3,4,5-Tetrabromofuran Munawar Hussain, a Rasheed Ahmad Khera, a Nguyen Thai Hung, a Peter Langer* a,b a Institut für Chemie, Universität Rostock,
PARTIAL NOTES for 6.1 Trigonometric Identities
 PARTIAL NOTES for 6.1 Trigonometric Identities tanθ = sinθ cosθ cotθ = cosθ sinθ BASIC IDENTITIES cscθ = 1 sinθ secθ = 1 cosθ cotθ = 1 tanθ PYTHAGOREAN IDENTITIES sin θ + cos θ =1 tan θ +1= sec θ 1 + cot
PARTIAL NOTES for 6.1 Trigonometric Identities tanθ = sinθ cosθ cotθ = cosθ sinθ BASIC IDENTITIES cscθ = 1 sinθ secθ = 1 cosθ cotθ = 1 tanθ PYTHAGOREAN IDENTITIES sin θ + cos θ =1 tan θ +1= sec θ 1 + cot
Aquinas College. Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET
 Aquinas College Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET Pearson Edexcel Level 3 Advanced Subsidiary and Advanced GCE in Mathematics and Further Mathematics Mathematical
Aquinas College Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET Pearson Edexcel Level 3 Advanced Subsidiary and Advanced GCE in Mathematics and Further Mathematics Mathematical
6.3 Forecasting ARMA processes
 122 CHAPTER 6. ARMA MODELS 6.3 Forecasting ARMA processes The purpose of forecasting is to predict future values of a TS based on the data collected to the present. In this section we will discuss a linear
122 CHAPTER 6. ARMA MODELS 6.3 Forecasting ARMA processes The purpose of forecasting is to predict future values of a TS based on the data collected to the present. In this section we will discuss a linear
Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3
 Supporting Information Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3 Shohei Shimokawa, Yuhsuke Kawagoe, Katsuhiko Moriyama, Hideo Togo* Graduate
Supporting Information Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3 Shohei Shimokawa, Yuhsuke Kawagoe, Katsuhiko Moriyama, Hideo Togo* Graduate
Exercises to Statistics of Material Fatigue No. 5
 Prof. Dr. Christine Müller Dipl.-Math. Christoph Kustosz Eercises to Statistics of Material Fatigue No. 5 E. 9 (5 a Show, that a Fisher information matri for a two dimensional parameter θ (θ,θ 2 R 2, can
Prof. Dr. Christine Müller Dipl.-Math. Christoph Kustosz Eercises to Statistics of Material Fatigue No. 5 E. 9 (5 a Show, that a Fisher information matri for a two dimensional parameter θ (θ,θ 2 R 2, can
VBA Microsoft Excel. J. Comput. Chem. Jpn., Vol. 5, No. 1, pp (2006)
 J. Comput. Chem. Jpn., Vol. 5, No. 1, pp. 29 38 (2006) Microsoft Excel, 184-8588 2-24-16 e-mail: yosimura@cc.tuat.ac.jp (Received: July 28, 2005; Accepted for publication: October 24, 2005; Published on
J. Comput. Chem. Jpn., Vol. 5, No. 1, pp. 29 38 (2006) Microsoft Excel, 184-8588 2-24-16 e-mail: yosimura@cc.tuat.ac.jp (Received: July 28, 2005; Accepted for publication: October 24, 2005; Published on
Supplementary materials. Mode Analysis. Matthias M. N. Wolf, Christian Schumann, Ruth Groß, Tatiana Domratcheva 1 and Rolf. Diller
 Supplementary materials Ultrafast Infrared Spectroscopy of Riboflavin: Dynamics, Electronic Structure, and Vibrational Mode Analysis Matthias M. N. Wolf, Christian Schumann, Ruth Groß, Tatiana Domratcheva
Supplementary materials Ultrafast Infrared Spectroscopy of Riboflavin: Dynamics, Electronic Structure, and Vibrational Mode Analysis Matthias M. N. Wolf, Christian Schumann, Ruth Groß, Tatiana Domratcheva
Supporting Information for. Update of spectroscopic data for 4-hydroxyldictyolactone and dictyol E isolated from a Halimeda stuposa - Dictyota
 1 Supporting Information for Update of spectroscopic data for 4-hydroxyldictyolactone and dictyol E isolated from a Halimeda stuposa - Dictyota sp. Assemblage Simon P. B. Ovenden, Jonathan L. Nielson,
1 Supporting Information for Update of spectroscopic data for 4-hydroxyldictyolactone and dictyol E isolated from a Halimeda stuposa - Dictyota sp. Assemblage Simon P. B. Ovenden, Jonathan L. Nielson,
k A = [k, k]( )[a 1, a 2 ] = [ka 1,ka 2 ] 4For the division of two intervals of confidence in R +
[a 1, a 2 ] = [ka 1,ka 2 ] 4For the division of two intervals of confidence in R + k A = [k, k]( )[a 1, a 2 ] = [ka 1,ka 2 ] 4For the division of two intervals of confidence in R +](/thumbs/73/69566903.jpg) Chapter 3. Fuzzy Arithmetic 3- Fuzzy arithmetic: ~Addition(+) and subtraction (-): Let A = [a and B = [b, b in R If x [a and y [b, b than x+y [a +b +b Symbolically,we write A(+)B = [a (+)[b, b = [a +b
Chapter 3. Fuzzy Arithmetic 3- Fuzzy arithmetic: ~Addition(+) and subtraction (-): Let A = [a and B = [b, b in R If x [a and y [b, b than x+y [a +b +b Symbolically,we write A(+)B = [a (+)[b, b = [a +b
Cite as: Pol Antras, course materials for International Economics I, Spring MIT OpenCourseWare (http://ocw.mit.edu/), Massachusetts
 / / σ/σ σ/σ θ θ θ θ y 1 0.75 0.5 0.25 0 0 0.5 1 1.5 2 θ θ θ x θ θ Φ θ Φ θ Φ π θ /Φ γφ /θ σ θ π θ Φ θ θ Φ θ θ θ θ σ θ / Φ θ θ / Φ / θ / θ Normalized import share: (Xni / Xn) / (XII / XI) 1 0.1 0.01 0.001
/ / σ/σ σ/σ θ θ θ θ y 1 0.75 0.5 0.25 0 0 0.5 1 1.5 2 θ θ θ x θ θ Φ θ Φ θ Φ π θ /Φ γφ /θ σ θ π θ Φ θ θ Φ θ θ θ θ σ θ / Φ θ θ / Φ / θ / θ Normalized import share: (Xni / Xn) / (XII / XI) 1 0.1 0.01 0.001
Heterobimetallic Pd-Sn Catalysis: Michael Addition. Reaction with C-, N-, O-, S- Nucleophiles and In-situ. Diagnostics
 Supporting Information (SI) Heterobimetallic Pd-Sn Catalysis: Michael Addition Reaction with C-, N-, -, S- Nucleophiles and In-situ Diagnostics Debjit Das, a Sanjay Pratihar a,b and Sujit Roy c * a rganometallics
Supporting Information (SI) Heterobimetallic Pd-Sn Catalysis: Michael Addition Reaction with C-, N-, -, S- Nucleophiles and In-situ Diagnostics Debjit Das, a Sanjay Pratihar a,b and Sujit Roy c * a rganometallics
CHAPTER 48 APPLICATIONS OF MATRICES AND DETERMINANTS
 CHAPTER 48 APPLICATIONS OF MATRICES AND DETERMINANTS EXERCISE 01 Page 545 1. Use matrices to solve: 3x + 4y x + 5y + 7 3x + 4y x + 5y 7 Hence, 3 4 x 0 5 y 7 The inverse of 3 4 5 is: 1 5 4 1 5 4 15 8 3
CHAPTER 48 APPLICATIONS OF MATRICES AND DETERMINANTS EXERCISE 01 Page 545 1. Use matrices to solve: 3x + 4y x + 5y + 7 3x + 4y x + 5y 7 Hence, 3 4 x 0 5 y 7 The inverse of 3 4 5 is: 1 5 4 1 5 4 15 8 3
Table of Contents 1 Supplementary Data MCD
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes
![Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes](/thumbs/92/108867714.jpg) SUPPORTING INFORMATION Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Eric J. Uzelac, Casey B. McCausland, and Seth C. Rasmussen*
SUPPORTING INFORMATION Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Eric J. Uzelac, Casey B. McCausland, and Seth C. Rasmussen*
Inverse trigonometric functions & General Solution of Trigonometric Equations. ------------------ ----------------------------- -----------------
 Inverse trigonometric functions & General Solution of Trigonometric Equations. 1. Sin ( ) = a) b) c) d) Ans b. Solution : Method 1. Ans a: 17 > 1 a) is rejected. w.k.t Sin ( sin ) = d is rejected. If sin
Inverse trigonometric functions & General Solution of Trigonometric Equations. 1. Sin ( ) = a) b) c) d) Ans b. Solution : Method 1. Ans a: 17 > 1 a) is rejected. w.k.t Sin ( sin ) = d is rejected. If sin
Synthesis, structural studies and stability of the model, cysteine containing DNA-protein cross-links
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 ELECTRONIC SUPPLEMENTARY INFORMATION
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 ELECTRONIC SUPPLEMENTARY INFORMATION
Problem Set 9 Solutions. θ + 1. θ 2 + cotθ ( ) sinθ e iφ is an eigenfunction of the ˆ L 2 operator. / θ 2. φ 2. sin 2 θ φ 2. ( ) = e iφ. = e iφ cosθ.
 Chemistry 362 Dr Jean M Standard Problem Set 9 Solutions The ˆ L 2 operator is defined as Verify that the angular wavefunction Y θ,φ) Also verify that the eigenvalue is given by 2! 2 & L ˆ 2! 2 2 θ 2 +
Chemistry 362 Dr Jean M Standard Problem Set 9 Solutions The ˆ L 2 operator is defined as Verify that the angular wavefunction Y θ,φ) Also verify that the eigenvalue is given by 2! 2 & L ˆ 2! 2 2 θ 2 +
Supplementary!Information!
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015! Synthesis)and)characterisation)of)an)open1cage) fullerene)encapsulating)hydrogen)fluoride! Supplementary!Information!
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015! Synthesis)and)characterisation)of)an)open1cage) fullerene)encapsulating)hydrogen)fluoride! Supplementary!Information!
Notes on the Open Economy
 Notes on the Open Econom Ben J. Heijdra Universit of Groningen April 24 Introduction In this note we stud the two-countr model of Table.4 in more detail. restated here for convenience. The model is Table.4.
Notes on the Open Econom Ben J. Heijdra Universit of Groningen April 24 Introduction In this note we stud the two-countr model of Table.4 in more detail. restated here for convenience. The model is Table.4.
ΕΙΣΑΓΩΓΗ ΣΤΗ ΣΤΑΤΙΣΤΙΚΗ ΑΝΑΛΥΣΗ
 ΕΙΣΑΓΩΓΗ ΣΤΗ ΣΤΑΤΙΣΤΙΚΗ ΑΝΑΛΥΣΗ ΕΛΕΝΑ ΦΛΟΚΑ Επίκουρος Καθηγήτρια Τµήµα Φυσικής, Τοµέας Φυσικής Περιβάλλοντος- Μετεωρολογίας ΓΕΝΙΚΟΙ ΟΡΙΣΜΟΙ Πληθυσµός Σύνολο ατόµων ή αντικειµένων στα οποία αναφέρονται
ΕΙΣΑΓΩΓΗ ΣΤΗ ΣΤΑΤΙΣΤΙΚΗ ΑΝΑΛΥΣΗ ΕΛΕΝΑ ΦΛΟΚΑ Επίκουρος Καθηγήτρια Τµήµα Φυσικής, Τοµέας Φυσικής Περιβάλλοντος- Μετεωρολογίας ΓΕΝΙΚΟΙ ΟΡΙΣΜΟΙ Πληθυσµός Σύνολο ατόµων ή αντικειµένων στα οποία αναφέρονται
SUPPLEMENTARY INFORMATION
 Sensitivity of [Ru(phen) 2 dppz] 2+ Light Switch Emission to Ionic Strength, Temperature, and DNA Sequence and Conformation Andrew W. McKinley, Per Lincoln and Eimer M. Tuite* SUPPLEMENTARY INFORMATION
Sensitivity of [Ru(phen) 2 dppz] 2+ Light Switch Emission to Ionic Strength, Temperature, and DNA Sequence and Conformation Andrew W. McKinley, Per Lincoln and Eimer M. Tuite* SUPPLEMENTARY INFORMATION
Supplementary figures
 A Supplementary figures a) DMT.BG2 0.87 0.87 0.72 20 40 60 80 100 DMT.EG2 0.93 0.85 20 40 60 80 EMT.MG3 0.85 0 20 40 60 80 20 40 60 80 100 20 40 60 80 100 20 40 60 80 EMT.G6 DMT/EMT b) EG2 0.92 0.85 5
A Supplementary figures a) DMT.BG2 0.87 0.87 0.72 20 40 60 80 100 DMT.EG2 0.93 0.85 20 40 60 80 EMT.MG3 0.85 0 20 40 60 80 20 40 60 80 100 20 40 60 80 100 20 40 60 80 EMT.G6 DMT/EMT b) EG2 0.92 0.85 5
ΤΜΗΜΑ ΦΥΣΙΚΩΝ ΠΟΡΩΝ & ΠΕΡΙΒΑΛΛΟΝΤΟΣ
 ΤΕΧΝΟΛΟΓΙΚΟ ΕΚΠΑΙ ΕΥΤΙΚΟ Ι ΡΥΜΑ ΚΡΗΤΗΣ ΠΑΡΑΡΤΗΜΑ ΧΑΝΙΩΝ ΤΜΗΜΑ ΦΥΣΙΚΩΝ ΠΟΡΩΝ & ΠΕΡΙΒΑΛΛΟΝΤΟΣ ΤΟΜΕΑΣ ΠΕΡΙΒΑΛΛΟΝΤΙΚΗΣ ΤΕΧΝΟΛΟΓΙΑΣ ΕΡΓΑΣΤΗΡΙΟ ΠΕΡΙΒΑΛΛΟΝΤΙΚΗΣ ΧΗΜΕΙΑΣ & ΒΙΟΧΗΜΙΚΩΝ ΙΕΡΓΑΣΙΩΝ ΠΤΥΧΙΑΚΗ ΕΡΓΑΣΙΑ
ΤΕΧΝΟΛΟΓΙΚΟ ΕΚΠΑΙ ΕΥΤΙΚΟ Ι ΡΥΜΑ ΚΡΗΤΗΣ ΠΑΡΑΡΤΗΜΑ ΧΑΝΙΩΝ ΤΜΗΜΑ ΦΥΣΙΚΩΝ ΠΟΡΩΝ & ΠΕΡΙΒΑΛΛΟΝΤΟΣ ΤΟΜΕΑΣ ΠΕΡΙΒΑΛΛΟΝΤΙΚΗΣ ΤΕΧΝΟΛΟΓΙΑΣ ΕΡΓΑΣΤΗΡΙΟ ΠΕΡΙΒΑΛΛΟΝΤΙΚΗΣ ΧΗΜΕΙΑΣ & ΒΙΟΧΗΜΙΚΩΝ ΙΕΡΓΑΣΙΩΝ ΠΤΥΧΙΑΚΗ ΕΡΓΑΣΙΑ
Parametrized Surfaces
 Parametrized Surfaces Recall from our unit on vector-valued functions at the beginning of the semester that an R 3 -valued function c(t) in one parameter is a mapping of the form c : I R 3 where I is some
Parametrized Surfaces Recall from our unit on vector-valued functions at the beginning of the semester that an R 3 -valued function c(t) in one parameter is a mapping of the form c : I R 3 where I is some
Απόκριση σε Μοναδιαία Ωστική Δύναμη (Unit Impulse) Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο. Απόστολος Σ.
 Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο The time integral of a force is referred to as impulse, is determined by and is obtained from: Newton s 2 nd Law of motion states that the action
Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο The time integral of a force is referred to as impulse, is determined by and is obtained from: Newton s 2 nd Law of motion states that the action
Supporting Information
 Supporting Information rigin of the Regio- and Stereoselectivity of Allylic Substitution of rganocopper Reagents Naohiko Yoshikai, Song-Lin Zhang, and Eiichi Nakamura* Department of Chemistry, The University
Supporting Information rigin of the Regio- and Stereoselectivity of Allylic Substitution of rganocopper Reagents Naohiko Yoshikai, Song-Lin Zhang, and Eiichi Nakamura* Department of Chemistry, The University
