Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]
|
|
|
- Μενέλαος Παπακωνσταντίνου
- 6 χρόνια πριν
- Προβολές:
Transcript
1 d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)]
2
3 Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic properties of Suva 9100 refrigerant [ASHRAE designation: R-410A (50/50)], have been developed and are presented here. These tables are based on extensive experimental measurements. Equations have been developed, based on the Martin-Hou equation of state, which represent the data with accuracy and consistency throughout the entire range of temperature, pressure, and density. Vapor enthalpy and entropy are calculated from the standard Martin-Hou equations. Additional equations have been developed for the calculation of saturated liquid enthalpy, latent enthalpy, and saturated liquid entropy, and are presented here. Physical Properties Chemical Formula CH 2 F 2 /CHF 2 CF 3 (50/50% by weight) Molecular Weight Boiling Point at One Atmosphere C ( F) Critical Temperature C ( F) K ( R) Critical Pressure kpa (abs) ( psia) Critical Density kg/m 3 (30.52 lb/ft 3 ) Critical Volume m 3 /kg ( ft 3 /lb) Units and Factors t = temperature in C T = temperature in K = C P = pressure in kilopascals absolute [kpa (abs)] v f = volume of saturated liquid in m 3 /kg v g = volume of saturated vapor in m 3 /kg V = volume of superheated vapor in m 3 /kg d f = 1/v f = density of saturated liquid in kg/m 3 d g = 1/v g = density of saturated vapor in kg/m 3 h f = enthalpy of saturated liquid in kj/kg h fg = enthalpy of vaporization in kj/kg h g = enthalpy of saturated vapor in kj/kg H = enthalpy of superheated vapor in kj/kg s f = entropy of saturated liquid in kj/(kg) (K) s g = entropy of saturated vapor in kj/(kg) (K) S = entropy of superheated vapor in kj/(kg) (K) C p = heat capacity at constant pressure in kj/(kg) ( C) C v = heat capacity at constant volume in kj/(kg) ( C) v s = velocity of sound in m/sec The gas constant, R = J/(mole) (K) for Suva 9100, R = kj/kg K One atmosphere = kpa Reference point for enthalpy and entropy: h f = 200 kj/kg at 0 C s f = 1 kj/kg K at 0 C Equations 1. Conversion FactorsSI Units to IP Units Properties listed in the following thermodynamic tables in SI units can be converted to I/P units using the conversion factors shown below. Please note that in converting enthalpy and entropy from SI to I/P units, a change in reference states must be included (from H = 200 and S = 1 at 0 C for SI units to H = 0 and S = 0 at 40 F for I/P units). In the conversion equation below, H (ref) and S (ref) are the saturated liquid enthalpy and entropy at 40 C. For Suva 9100, H (ref) = kj/kg and S (ref) = kj/kg K. P (psia) = P (kpa [abs]) T ( F) = (T[ C] 1.8) + 32 D (lb/ft 3 ) = D (kg/m 3 ) V (ft 3 /lb) = V (m 3 /kg) H (Btu/lb) = [H (kj/kg) H (ref)] S (Btu/lb R) = [S (kj/kg K) S (ref)] C p (Btu/lb F) = C p (kj/kg K) C v (Btu/lb F) = C v (kj/kg K) v s (ft/sec) = v s (m/sec) Martin-Hou Equation of State Coefficients for the Martin-Hou equation of state are presented below: 5 P = RT/(V b) + Σ (A i + B i T + C i exp [ kt/t c ])/(V b) i i=2 1
4 For SI units T and T c are in K = C , V is in m 3 /kg, and P is in kpa (abs). R = kj/kg K for Suva 9100 b, A i, B i, C i, and k are constants: A 2 = E 01 A 4 = E 07 B 2 = E 04 B 4 = E+00 C 2 = E+00 C 4 = E+00 A 3 = E 04 A 5 = E 10 B 3 = E 08 B 5 = E 12 C 3 = E 02 C 5 = E 07 b = E 04 k = E+00 X and Y are constants used in the vapor enthalpy and entropy equations for the Martin-Hou equation of state: X = E+02 Y = E 01 For I/P units T and T c are in R = F , V is in ft 3 /lb, and P is in psia. R = (psia) (ft 3 )/lb R for Suva 9100 b, A i, B i, C i, and k are constants: A 2 = E+00 A 4 = E 03 B 2 = E 03 B 4 = E+00 C 2 = E+02 C 4 = E+00 A 3 = E 01 A 5 = E 05 B 3 = E 06 B 5 = E 07 C 3 = E+00 C 5 = E 02 b = E 03 k = E+00 X and Y are constants used in the vapor enthalpy and entropy equations for the Martin-Hou equation of state: X = E+01 Y = E 02 Ideal Gas Heat Capacity (at constant pressure): C o p = a + bt + ct 2 + dt 3 Ideal Gas Heat Capacity (at constant volume): C o v = C o p R For SI units C o p and C o v = kj/kg K R = kj/kg K for Suva 9100 T is in K = C a, b, c, d are constants: a = E 01 c = E 07 b = E 03 d = E 11 For I/P units C o p and C o v = Btu/lb R R = Btu/lb R for Suva 9100 T is in R = F a, b, c, d, are constants: a = E 02 c = E 08 b = E 04 d = E Liquid Enthalpy, Latent Enthalpy and Liquid Entropy Equations Saturated Liquid Enthalpy: h f = A + B X + C (X) 2 + D (X) 3 + E (X) 4 + F (X) 5 where X = (1 T r ) 1/3 X o, and T r = T/T c Latent Enthalpy: h fg = h g h f Saturated Liquid Entropy: s f = s g ([h g h f ]/T) For SI units h f, h g, and h fg are in kj/kg s f and s g are in kj/(kg) (K) T and T c are in K = C A, B, C, D, E, F, and X o are constants: A = E+02 D = E+02 B = E+02 E = E+03 C = E+02 F = E+03 X o = E 01 2
5 For I/P units h f, h g, and h fg are in Btu/lb s f and s g are in Btu/(lb) ( R) T and T c are in R = F A, B, C, D, E, F, and X o are constants: A = E+01 D = E+02 B = E+02 E = E+02 C = E+02 F = E+02 X o = E Vapor Pressure log n (P sat /P c ) = 1/T r (A + B X + C X 2 + D X 3 + E X 4 + F X 5 ) where X = (1 T r ) X o, and T r = T/T c A, B, C, D, E, F, and X o are constants: Constants for vapor pressure of saturated liquid (bubble point), p f : A = E+00 D = E+00 B = E+00 E = E+00 C = E 01 F = E+00 X o = E 01 Constants for vapor pressure of saturated vapor (dew point), p g : A = E+00 D = E+00 B = E+00 E = E+00 C = E 01 F = E+00 X o = E 01 Because both pressure and temperature appear in the reduced form in the equation, the same constants can be used for either SI or I/P units. For SI units T and T c are in K = C P and P c are in kpa (abs) For I/P units T and T c are in R = F P and P c are in psia 4. Density of the Saturated Liquid d f /Dc = A f + B f (1 T r ) (1/3) + C f (1 T r ) (2/3) + D f (1 T r ) + E f (1 T r ) (4/3) A f, B f, C f, D f, E f are constants: A f = E+00 D f = E+00 B f = E+00 E f = E 01 C f = E 01 Because both density and temperature appear in the reduced form in the equation, the same constants can be used for either SI or I/P units. For SI units T r and T/T c, both in K = C d f and D c are in kg/m 3 For I/P units T r and T/T c, both in R = F d f and D c are in lb/ft 3 3
6 TABLE 1 SUVA 9100 Saturation PropertiesTemperature Table C LIQUID p f PRESSURE kpa p g LIQUID v f VOLUME m 3 /kg v g DENSITY kg/m 3 LIQUID LIQUID 1/v f 1/v g h f ENTHALPY kj/kg LATENT h fg h g LIQUID s f ENTROPY kj/(kg)(k) s g C
7 C LIQUID p f PRESSURE kpa TABLE 1 (continued) SUVA 9100 Saturation PropertiesTemperature Table p g LIQUID v f VOLUME m 3 /kg v g DENSITY kg/m 3 LIQUID LIQUID 1/v f 1/v g h f ENTHALPY kj/kg LATENT h fg h g LIQUID s f ENTROPY kj/(kg)(k) s g C 5
8 C LIQUID p f PRESSURE kpa TABLE 1 (continued) SUVA 9100 Saturation PropertiesTemperature Table p g LIQUID v f VOLUME m 3 /kg v g DENSITY kg/m 3 LIQUID LIQUID 1/v f 1/v g h f ENTHALPY kj/kg LATENT h fg h g LIQUID s f ENTROPY kj/(kg)(k) s g C 6
9 TABLE 2 SUVA 9100 Superheated VaporConstant Pressure Tables V = Volume in m 3 /kg H = Enthalpy in kj/kg S = Entropy in kj/(kg) (K) (Saturated Vapor Properties in parentheses) ABSOLUTE PRESSURE, kpa ( C) ( C) ( C) ( C) (2.0972) (381.4) (2.1020) (1.0953) (386.8) (2.0518) (0.7491) (390.2) (2.0234) (0.5721) (392.7) (2.0036) ( C) ( C) ( C) ( C) (0.4641) (394.7) (1.9885) (0.3911) (396.4) (1.9764) (0.3384) (397.9) (1.9662) (0.2985) (399.1) (1.9574)
10 TABLE 2 (continued) SUVA 9100 Superheated VaporConstant Pressure Tables V = Volume in m 3 /kg H = Enthalpy in kj/kg S = Entropy in kj/(kg) (K) (Saturated Vapor Properties in parentheses) ABSOLUTE PRESSURE, kpa ( C) ( C) ( C) ( C) (0.2672) (400.3) (1.9498) (0.2420) (401.3) (1.9430) (0.2390) (401.5) (1.9421) (0.2212) (402.3) (1.9369) ( C) ( C) ( C) ( C) (0.2037) (403.2) (1.9313) (0.1889) (404.0) (1.9262) (0.1761) (404.7) (1.9215) (0.1650) (405.5) (1.9172)
11 TABLE 2 (continued) SUVA 9100 Superheated VaporConstant Pressure Tables V = Volume in m 3 /kg H = Enthalpy in kj/kg S = Entropy in kj/(kg) (K) (Saturated Vapor Properties in parentheses) ABSOLUTE PRESSURE, kpa ( C) ( C) ( C) ( C) (0.1552) (406.1) (1.9131) (0.1465) (406.7) (1.9093) (0.1388) (407.3) (1.9058) (0.1318) (407.9) (1.9024) ( C) ( C) ( C) ( C) (0.1255) (408.4) (1.8992) (0.1198) (409.0) (1.8962) (0.1146) (409.4) (1.8933) (0.1099) (409.9) (1.8906)
12 TABLE 2 (continued) SUVA 9100 Superheated VaporConstant Pressure Tables V = Volume in m 3 /kg H = Enthalpy in kj/kg S = Entropy in kj/(kg) (K) (Saturated Vapor Properties in parentheses) ABSOLUTE PRESSURE, kpa ( C) ( C) ( C) ( C) (0.1055) (410.4) (1.8880) (0.1015) (410.8) (1.8854) (0.0977) (411.2) (1.8830) (0.0943) (411.6) (1.8807) ( C) ( C) ( C) ( C) (0.0910) (412.0) (1.8785) (0.0880) (412.4) (1.8764) (0.0852) (412.7) (1.8743) (0.0825) (413.1) (1.8723)
13 TABLE 2 (continued) SUVA 9100 Superheated VaporConstant Pressure Tables V = Volume in m 3 /kg H = Enthalpy in kj/kg S = Entropy in kj/(kg) (K) (Saturated Vapor Properties in parentheses) ABSOLUTE PRESSURE, kpa ( C) ( C) ( C) ( C) (0.0800) (413.4) (1.8703) (0.0777) (413.7) (1.8685) (0.0755) (414.0) (1.8667) (0.0734) (414.3) (1.8649) ( C) ( C) ( C) ( C) (0.0714) (414.6) (1.8632) (0.0696) (414.9) (1.8615) (0.0678) (415.2) (1.8599) (0.0661) (415.5) (1.8583)
DuPont Suva 95 Refrigerant
 Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
DuPont Suva 95 Refrigerant
 Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
DuPont Suva. DuPont. Thermodynamic Properties of. Refrigerant (R-410A) Technical Information. refrigerants T-410A ENG
 Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
ddupont Fluorochemicals
 Technical Information T-23 ENG ddupont Fluorochemicals Thermodynamic Properties of HFC-23 (trifluoromethane) DuPont Product Names: Freon 23 Thermodynamic Properties of HFC-23 Refrigerant (trifluoromethane)
Technical Information T-23 ENG ddupont Fluorochemicals Thermodynamic Properties of HFC-23 (trifluoromethane) DuPont Product Names: Freon 23 Thermodynamic Properties of HFC-23 Refrigerant (trifluoromethane)
STEAM TABLES. Mollier Diagram
 STEAM TABLES and Mollier Diagram (S.I. Units) dharm \M-therm\C-steam.pm5 CONTENTS Table No. Page No. 1. Saturated Water and Steam (Temperature) Tables I (ii) 2. Saturated Water and Steam (Pressure) Tables
STEAM TABLES and Mollier Diagram (S.I. Units) dharm \M-therm\C-steam.pm5 CONTENTS Table No. Page No. 1. Saturated Water and Steam (Temperature) Tables I (ii) 2. Saturated Water and Steam (Pressure) Tables
PROPERTIES OF ORDINARY WATER SUBSTANCE AT SATURATION FROF TME CRITICAL POINT DOWN TO 66 C EQUATIONS & TABLES
 PROPERTIES OF ORDINARY WATER SUBSTANCE AT SATURATION FROF TME CRITICAL POINT DOWN TO 66 C EQUATIONS & TABLES M. CONDE ENGINEERING, 2002 M. CONDE ENGINEERING, Zurich 2002 Properties of ordinary water substance
PROPERTIES OF ORDINARY WATER SUBSTANCE AT SATURATION FROF TME CRITICAL POINT DOWN TO 66 C EQUATIONS & TABLES M. CONDE ENGINEERING, 2002 M. CONDE ENGINEERING, Zurich 2002 Properties of ordinary water substance
Ύγρανση και Αφύγρανση. Ψυχρομετρία. 21-Nov-16
 Ύγρανση και Αφύγρανση Ψυχρομετρία η μελέτη των ιδιοτήτων του υγρού αέρα δηλαδή του μίγματος αέρα-ατμού-νερού. Ένα βασικό πρόβλημα: Δεδομένων των P (bar) Πίεση T ( o C) Θερμοκρασία Υ (kg/kg ξβ) Υγρασία
Ύγρανση και Αφύγρανση Ψυχρομετρία η μελέτη των ιδιοτήτων του υγρού αέρα δηλαδή του μίγματος αέρα-ατμού-νερού. Ένα βασικό πρόβλημα: Δεδομένων των P (bar) Πίεση T ( o C) Θερμοκρασία Υ (kg/kg ξβ) Υγρασία
By R.L. Snyder (Revised March 24, 2005)
 Humidity Conversion By R.L. Snyder (Revised March 24, 2005) This Web page provides the equations used to make humidity conversions and tables o saturation vapor pressure. For a pd ile o this document,
Humidity Conversion By R.L. Snyder (Revised March 24, 2005) This Web page provides the equations used to make humidity conversions and tables o saturation vapor pressure. For a pd ile o this document,
Thermodynamic Tables to accompany Modern Engineering Thermodynamics
 Thermodynamic Tables to accompany Modern Engineering Thermodynamics This page intentionally left blank Thermodynamic Tables to accompany Modern Engineering Thermodynamics Robert T. Balmer AMSTERDAM BOSTON
Thermodynamic Tables to accompany Modern Engineering Thermodynamics This page intentionally left blank Thermodynamic Tables to accompany Modern Engineering Thermodynamics Robert T. Balmer AMSTERDAM BOSTON
Mean bond enthalpy Standard enthalpy of formation Bond N H N N N N H O O O
 Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Pof moist air and uses these properties to analyze conditions and
 CHAPTER 6 PSYCHROMETRICS Composition of Dry and Moist Air... 6.1 United States Standard Atmosphere... 6.1 Thermodynamic Properties of Moist Air... 6.2 Thermodynamic Properties of Water at Saturation...
CHAPTER 6 PSYCHROMETRICS Composition of Dry and Moist Air... 6.1 United States Standard Atmosphere... 6.1 Thermodynamic Properties of Moist Air... 6.2 Thermodynamic Properties of Water at Saturation...
Figure 1 T / K Explain, in terms of molecules, why the first part of the graph in Figure 1 is a line that slopes up from the origin.
 Q1.(a) Figure 1 shows how the entropy of a molecular substance X varies with temperature. Figure 1 T / K (i) Explain, in terms of molecules, why the entropy is zero when the temperature is zero Kelvin.
Q1.(a) Figure 1 shows how the entropy of a molecular substance X varies with temperature. Figure 1 T / K (i) Explain, in terms of molecules, why the entropy is zero when the temperature is zero Kelvin.
APPENDIX A. Summary of the English Engineering (EE) System of Units
 Appendixes A. Summary of the English Engineering (EE) System of Units B. Summary of the International System (SI) of Units C. Friction-Factor Chart D. Oblique-Shock Charts (γ = 1.4) (Two-Dimensional) E.
Appendixes A. Summary of the English Engineering (EE) System of Units B. Summary of the International System (SI) of Units C. Friction-Factor Chart D. Oblique-Shock Charts (γ = 1.4) (Two-Dimensional) E.
Contents of Appendix
 Contents of Appendix A SI UNITS: SINGLE-STATE PROPERTIES 755 Table A.1 Conversion Factors, 755 Table A.2 Critical Constants, 758 Table A.3 Properties of Selected Solids at 25 C, 759 Table A.4 Properties
Contents of Appendix A SI UNITS: SINGLE-STATE PROPERTIES 755 Table A.1 Conversion Factors, 755 Table A.2 Critical Constants, 758 Table A.3 Properties of Selected Solids at 25 C, 759 Table A.4 Properties
Correction Table for an Alcoholometer Calibrated at 20 o C
 An alcoholometer is a device that measures the concentration of ethanol in a water-ethanol mixture (often in units of %abv percent alcohol by volume). The depth to which an alcoholometer sinks in a water-ethanol
An alcoholometer is a device that measures the concentration of ethanol in a water-ethanol mixture (often in units of %abv percent alcohol by volume). The depth to which an alcoholometer sinks in a water-ethanol
PETROSKILLS COPYRIGHT
 Contents Solution Gas-Oil Ratio... 2... 2... 2... 2 Formation Volume Factor... 3... 3... 3... 3 Viscosity... 4... 4... 4... 4 Density... 5 Bubble Point Pressure... 5... 6... 6... 6 Compressibility... 6
Contents Solution Gas-Oil Ratio... 2... 2... 2... 2 Formation Volume Factor... 3... 3... 3... 3 Viscosity... 4... 4... 4... 4 Density... 5 Bubble Point Pressure... 5... 6... 6... 6 Compressibility... 6
PETROSKILLS COPYRIGHT
 Contents Dew Point... 2 SI Conversions... 2 Output... 2 Input... 2 Solution Condensate-Oil Ratio... 3 SI Conversions... 3 Output... 3 Input... 3 Gas Density... 4 SI Conversions... 5 Output... 5 Input...
Contents Dew Point... 2 SI Conversions... 2 Output... 2 Input... 2 Solution Condensate-Oil Ratio... 3 SI Conversions... 3 Output... 3 Input... 3 Gas Density... 4 SI Conversions... 5 Output... 5 Input...
Approximation of distance between locations on earth given by latitude and longitude
 Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
DESIGN OF MACHINERY SOLUTION MANUAL h in h 4 0.
 DESIGN OF MACHINERY SOLUTION MANUAL -7-1! PROBLEM -7 Statement: Design a double-dwell cam to move a follower from to 25 6, dwell for 12, fall 25 and dwell for the remader The total cycle must take 4 sec
DESIGN OF MACHINERY SOLUTION MANUAL -7-1! PROBLEM -7 Statement: Design a double-dwell cam to move a follower from to 25 6, dwell for 12, fall 25 and dwell for the remader The total cycle must take 4 sec
2. Chemical Thermodynamics and Energetics - I
 . Chemical Thermodynamics and Energetics - I 1. Given : Initial Volume ( = 5L dm 3 Final Volume (V = 10L dm 3 ext = 304 cm of Hg Work done W = ext V ext = 304 cm of Hg = 304 atm [... 76cm of Hg = 1 atm]
. Chemical Thermodynamics and Energetics - I 1. Given : Initial Volume ( = 5L dm 3 Final Volume (V = 10L dm 3 ext = 304 cm of Hg Work done W = ext V ext = 304 cm of Hg = 304 atm [... 76cm of Hg = 1 atm]
Phys460.nb Solution for the t-dependent Schrodinger s equation How did we find the solution? (not required)
 Phys460.nb 81 ψ n (t) is still the (same) eigenstate of H But for tdependent H. The answer is NO. 5.5.5. Solution for the tdependent Schrodinger s equation If we assume that at time t 0, the electron starts
Phys460.nb 81 ψ n (t) is still the (same) eigenstate of H But for tdependent H. The answer is NO. 5.5.5. Solution for the tdependent Schrodinger s equation If we assume that at time t 0, the electron starts
PETROSKILLS COPYRIGHT
 Contents Specific Gravity... 2 Formation Volume Factor... 3 Compressibility Equation... 4 Pseudo-Reduced Variables... 5 Pseudo-Critical Variables... 6 SI Conversions... 6 Output... 6 Input... 6 Viscosity...
Contents Specific Gravity... 2 Formation Volume Factor... 3 Compressibility Equation... 4 Pseudo-Reduced Variables... 5 Pseudo-Critical Variables... 6 SI Conversions... 6 Output... 6 Input... 6 Viscosity...
CHAPTER 25 SOLVING EQUATIONS BY ITERATIVE METHODS
 CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
UDZ Swirl diffuser. Product facts. Quick-selection. Swirl diffuser UDZ. Product code example:
 UDZ Swirl diffuser Swirl diffuser UDZ, which is intended for installation in a ventilation duct, can be used in premises with a large volume, for example factory premises, storage areas, superstores, halls,
UDZ Swirl diffuser Swirl diffuser UDZ, which is intended for installation in a ventilation duct, can be used in premises with a large volume, for example factory premises, storage areas, superstores, halls,
Συστήματα Βιομηχανικών Διεργασιών 6ο εξάμηνο
 Συστήματα Βιομηχανικών Διεργασιών 6ο εξάμηνο Μέρος 1 ο : Εισαγωγικά (διαστ., πυκν., θερμ., πίεση, κτλ.) Μέρος 2 ο : Ισοζύγια μάζας Μέρος 3 ο : 8 ο μάθημα Εκτός ύλης ΔΠΘ-ΜΠΔ Συστήματα Βιομηχανικών Διεργασιών
Συστήματα Βιομηχανικών Διεργασιών 6ο εξάμηνο Μέρος 1 ο : Εισαγωγικά (διαστ., πυκν., θερμ., πίεση, κτλ.) Μέρος 2 ο : Ισοζύγια μάζας Μέρος 3 ο : 8 ο μάθημα Εκτός ύλης ΔΠΘ-ΜΠΔ Συστήματα Βιομηχανικών Διεργασιών
6.1. Dirac Equation. Hamiltonian. Dirac Eq.
 6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
Homework 3 Solutions
 Homework 3 Solutions Igor Yanovsky (Math 151A TA) Problem 1: Compute the absolute error and relative error in approximations of p by p. (Use calculator!) a) p π, p 22/7; b) p π, p 3.141. Solution: For
Homework 3 Solutions Igor Yanovsky (Math 151A TA) Problem 1: Compute the absolute error and relative error in approximations of p by p. (Use calculator!) a) p π, p 22/7; b) p π, p 3.141. Solution: For
IV. ANHANG 179. Anhang 178
 Anhang 178 IV. ANHANG 179 1. Röntgenstrukturanalysen (Tabellen) 179 1.1. Diastereomer A (Diplomarbeit) 179 1.2. Diastereomer B (Diplomarbeit) 186 1.3. Aldoladdukt 5A 193 1.4. Aldoladdukt 13A 200 1.5. Aldoladdukt
Anhang 178 IV. ANHANG 179 1. Röntgenstrukturanalysen (Tabellen) 179 1.1. Diastereomer A (Diplomarbeit) 179 1.2. Diastereomer B (Diplomarbeit) 186 1.3. Aldoladdukt 5A 193 1.4. Aldoladdukt 13A 200 1.5. Aldoladdukt
ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΤΜΗΜΑ ΧΗΜΙΚΩΝ ΜΗΧΑΝΙΚΩΝ ΕΡΓΑΣΤΗΡΙΟ ΣΧΕΔΙΑΣΜΟΥ & ΑΝΑΛΥΣΗΣ ΔΙΕΡΓΑΣΙΩΝ
 ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΤΜΗΜΑ ΧΗΜΙΚΩΝ ΜΗΧΑΝΙΚΩΝ ΕΡΓΑΣΤΗΡΙΟ ΣΧΕΔΙΑΣΜΟΥ & ΑΝΑΛΥΣΗΣ ΔΙΕΡΓΑΣΙΩΝ Μάθημα: ΤΕΧΝΙΚΗ ΦΥΣΙΚΩΝ ΔΙΕΡΓΑΣΙΩΝ ΙΙ 6ο Εξάμηνο Χημικών Μηχανικών ΞΗΡΑΝΤΗΡΕΣ 1 Characteristics of Food Dryers
ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΤΜΗΜΑ ΧΗΜΙΚΩΝ ΜΗΧΑΝΙΚΩΝ ΕΡΓΑΣΤΗΡΙΟ ΣΧΕΔΙΑΣΜΟΥ & ΑΝΑΛΥΣΗΣ ΔΙΕΡΓΑΣΙΩΝ Μάθημα: ΤΕΧΝΙΚΗ ΦΥΣΙΚΩΝ ΔΙΕΡΓΑΣΙΩΝ ΙΙ 6ο Εξάμηνο Χημικών Μηχανικών ΞΗΡΑΝΤΗΡΕΣ 1 Characteristics of Food Dryers
Second Order RLC Filters
 ECEN 60 Circuits/Electronics Spring 007-0-07 P. Mathys Second Order RLC Filters RLC Lowpass Filter A passive RLC lowpass filter (LPF) circuit is shown in the following schematic. R L C v O (t) Using phasor
ECEN 60 Circuits/Electronics Spring 007-0-07 P. Mathys Second Order RLC Filters RLC Lowpass Filter A passive RLC lowpass filter (LPF) circuit is shown in the following schematic. R L C v O (t) Using phasor
Math 6 SL Probability Distributions Practice Test Mark Scheme
 Math 6 SL Probability Distributions Practice Test Mark Scheme. (a) Note: Award A for vertical line to right of mean, A for shading to right of their vertical line. AA N (b) evidence of recognizing symmetry
Math 6 SL Probability Distributions Practice Test Mark Scheme. (a) Note: Award A for vertical line to right of mean, A for shading to right of their vertical line. AA N (b) evidence of recognizing symmetry
3.4 SUM AND DIFFERENCE FORMULAS. NOTE: cos(α+β) cos α + cos β cos(α-β) cos α -cos β
 3.4 SUM AND DIFFERENCE FORMULAS Page Theorem cos(αβ cos α cos β -sin α cos(α-β cos α cos β sin α NOTE: cos(αβ cos α cos β cos(α-β cos α -cos β Proof of cos(α-β cos α cos β sin α Let s use a unit circle
3.4 SUM AND DIFFERENCE FORMULAS Page Theorem cos(αβ cos α cos β -sin α cos(α-β cos α cos β sin α NOTE: cos(αβ cos α cos β cos(α-β cos α -cos β Proof of cos(α-β cos α cos β sin α Let s use a unit circle
titanium laser beam volume to be heated joint titanium Fig. 5.1
 1 Lasers are often used to form precision-welded joints in titanium. To form one such joint it is first necessary to increase the temperature of the titanium to its melting point. Fig. 5.1 shows the joint
1 Lasers are often used to form precision-welded joints in titanium. To form one such joint it is first necessary to increase the temperature of the titanium to its melting point. Fig. 5.1 shows the joint
Suva refrigerants. Transport Properties of Suva 410A Refrigerant. Viscosity Thermal Conductivity and Heat Capacity for the Liquid and Vapor
 Product Information Suva refrigerants ART - 31 Transport Properties of Suva 410A Refrigerant Viscosity Thermal Conductivity and Heat Capacity for the Liquid and Vapor Transport Properties of Suva 410A
Product Information Suva refrigerants ART - 31 Transport Properties of Suva 410A Refrigerant Viscosity Thermal Conductivity and Heat Capacity for the Liquid and Vapor Transport Properties of Suva 410A
Suva. refrigerants. Transport Properties of Suva 407C Refrigerant. Viscosity Thermal Conductivity and Heat Capacity for the Liquid and Vapor
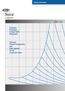 Product Information Suva refrigerants ART - 26 Transport Properties of Suva 407C Refrigerant Viscosity Thermal Conductivity and Heat Capacity for the Liquid and Vapor Transport Properties of Suva 407C
Product Information Suva refrigerants ART - 26 Transport Properties of Suva 407C Refrigerant Viscosity Thermal Conductivity and Heat Capacity for the Liquid and Vapor Transport Properties of Suva 407C
2 Composition. Invertible Mappings
 Arkansas Tech University MATH 4033: Elementary Modern Algebra Dr. Marcel B. Finan Composition. Invertible Mappings In this section we discuss two procedures for creating new mappings from old ones, namely,
Arkansas Tech University MATH 4033: Elementary Modern Algebra Dr. Marcel B. Finan Composition. Invertible Mappings In this section we discuss two procedures for creating new mappings from old ones, namely,
Magnet Wire General Engineering Data Bare and Film Insulated Copper and Aluminum
 Magnet Wire General Engineering Data Bare and Film Insulated Copper and Aluminum CABLE Magnet Wire General Engineering Data Bare and Film Insulated Copper and Aluminum Forward This booklet contains engineering
Magnet Wire General Engineering Data Bare and Film Insulated Copper and Aluminum CABLE Magnet Wire General Engineering Data Bare and Film Insulated Copper and Aluminum Forward This booklet contains engineering
An experimental and theoretical study of the gas phase kinetics of atomic chlorine reactions with CH 3 NH 2, (CH 3 ) 2 NH, and (CH 3 ) 3 N
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
Problem Set 9 Solutions. θ + 1. θ 2 + cotθ ( ) sinθ e iφ is an eigenfunction of the ˆ L 2 operator. / θ 2. φ 2. sin 2 θ φ 2. ( ) = e iφ. = e iφ cosθ.
 Chemistry 362 Dr Jean M Standard Problem Set 9 Solutions The ˆ L 2 operator is defined as Verify that the angular wavefunction Y θ,φ) Also verify that the eigenvalue is given by 2! 2 & L ˆ 2! 2 2 θ 2 +
Chemistry 362 Dr Jean M Standard Problem Set 9 Solutions The ˆ L 2 operator is defined as Verify that the angular wavefunction Y θ,φ) Also verify that the eigenvalue is given by 2! 2 & L ˆ 2! 2 2 θ 2 +
Selecting Critical Properties of Terpenes and Terpenoids through Group-Contribution Methods and Equations of State
 Supporting Information Selecting Critical Properties of Terpenes and Terpenoids through Group-Contribution Methods and Equations of State Mónia A. R. Martins, 1,2 Pedro J. Carvalho, 1 André M. Palma, 1
Supporting Information Selecting Critical Properties of Terpenes and Terpenoids through Group-Contribution Methods and Equations of State Mónia A. R. Martins, 1,2 Pedro J. Carvalho, 1 André M. Palma, 1
Assalamu `alaikum wr. wb.
 LUMP SUM Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. LUMP SUM Lump sum lump sum lump sum. lump sum fixed price lump sum lump
LUMP SUM Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. LUMP SUM Lump sum lump sum lump sum. lump sum fixed price lump sum lump
4 Way Reversing Valve
 STANDARD 4 Way Reversing Valve SHF series four-way reversing valves are applicable for heat pump systems such as central, unitary and room air conditioners to realize switching between cooling mode and
STANDARD 4 Way Reversing Valve SHF series four-way reversing valves are applicable for heat pump systems such as central, unitary and room air conditioners to realize switching between cooling mode and
Aluminum Electrolytic Capacitors (Large Can Type)
 Aluminum Electrolytic Capacitors (Large Can Type) Snap-In, 85 C TS-U ECE-S (U) Series: TS-U Features General purpose Wide CV value range (33 ~ 47,000 µf/16 4V) Various case sizes Top vent construction
Aluminum Electrolytic Capacitors (Large Can Type) Snap-In, 85 C TS-U ECE-S (U) Series: TS-U Features General purpose Wide CV value range (33 ~ 47,000 µf/16 4V) Various case sizes Top vent construction
Heat exchanger. Type WT. For the reheating of airflows in rectangular ducting PD WT 1. 03/2017 DE/en
 X X testregistrierung Heat exchanger Type For the reheating of airflows in rectangular ducting Rectangular hot water heat exchanger for the reheating of airflows, suitable for VAV terminal units Type TVR,
X X testregistrierung Heat exchanger Type For the reheating of airflows in rectangular ducting Rectangular hot water heat exchanger for the reheating of airflows, suitable for VAV terminal units Type TVR,
Exercises to Statistics of Material Fatigue No. 5
 Prof. Dr. Christine Müller Dipl.-Math. Christoph Kustosz Eercises to Statistics of Material Fatigue No. 5 E. 9 (5 a Show, that a Fisher information matri for a two dimensional parameter θ (θ,θ 2 R 2, can
Prof. Dr. Christine Müller Dipl.-Math. Christoph Kustosz Eercises to Statistics of Material Fatigue No. 5 E. 9 (5 a Show, that a Fisher information matri for a two dimensional parameter θ (θ,θ 2 R 2, can
Strain gauge and rosettes
 Strain gauge and rosettes Introduction A strain gauge is a device which is used to measure strain (deformation) on an object subjected to forces. Strain can be measured using various types of devices classified
Strain gauge and rosettes Introduction A strain gauge is a device which is used to measure strain (deformation) on an object subjected to forces. Strain can be measured using various types of devices classified
Dr. D. Dinev, Department of Structural Mechanics, UACEG
 Lecture 4 Material behavior: Constitutive equations Field of the game Print version Lecture on Theory of lasticity and Plasticity of Dr. D. Dinev, Department of Structural Mechanics, UACG 4.1 Contents
Lecture 4 Material behavior: Constitutive equations Field of the game Print version Lecture on Theory of lasticity and Plasticity of Dr. D. Dinev, Department of Structural Mechanics, UACG 4.1 Contents
Major Concepts. Multiphase Equilibrium Stability Applications to Phase Equilibrium. Two-Phase Coexistence
 Major Concepts Multiphase Equilibrium Stability Applications to Phase Equilibrium Phase Rule Clausius-Clapeyron Equation Special case of Gibbs-Duhem wo-phase Coexistence Criticality Metastability Spinodal
Major Concepts Multiphase Equilibrium Stability Applications to Phase Equilibrium Phase Rule Clausius-Clapeyron Equation Special case of Gibbs-Duhem wo-phase Coexistence Criticality Metastability Spinodal
Homework 8 Model Solution Section
 MATH 004 Homework Solution Homework 8 Model Solution Section 14.5 14.6. 14.5. Use the Chain Rule to find dz where z cosx + 4y), x 5t 4, y 1 t. dz dx + dy y sinx + 4y)0t + 4) sinx + 4y) 1t ) 0t + 4t ) sinx
MATH 004 Homework Solution Homework 8 Model Solution Section 14.5 14.6. 14.5. Use the Chain Rule to find dz where z cosx + 4y), x 5t 4, y 1 t. dz dx + dy y sinx + 4y)0t + 4) sinx + 4y) 1t ) 0t + 4t ) sinx
D Alembert s Solution to the Wave Equation
 D Alembert s Solution to the Wave Equation MATH 467 Partial Differential Equations J. Robert Buchanan Department of Mathematics Fall 2018 Objectives In this lesson we will learn: a change of variable technique
D Alembert s Solution to the Wave Equation MATH 467 Partial Differential Equations J. Robert Buchanan Department of Mathematics Fall 2018 Objectives In this lesson we will learn: a change of variable technique
Appendix to On the stability of a compressible axisymmetric rotating flow in a pipe. By Z. Rusak & J. H. Lee
 Appendi to On the stability of a compressible aisymmetric rotating flow in a pipe By Z. Rusak & J. H. Lee Journal of Fluid Mechanics, vol. 5 4, pp. 5 4 This material has not been copy-edited or typeset
Appendi to On the stability of a compressible aisymmetric rotating flow in a pipe By Z. Rusak & J. H. Lee Journal of Fluid Mechanics, vol. 5 4, pp. 5 4 This material has not been copy-edited or typeset
Si + Al Mg Fe + Mn +Ni Ca rim Ca p.f.u
 .6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
.6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
HOMEWORK 4 = G. In order to plot the stress versus the stretch we define a normalized stretch:
 HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
PETROSKILLS COPYRIGHT
 Contents Density (Standard Conditions)... 2... 2... 2... 2 Formation Volume Factor... 2... 3... 3... 3 Density (In-Situ)... 3 Compressibility... 3... 4... 4... 4 Viscosity... 4... 5... 5... 5 Viscosibility...
Contents Density (Standard Conditions)... 2... 2... 2... 2 Formation Volume Factor... 2... 3... 3... 3 Density (In-Situ)... 3 Compressibility... 3... 4... 4... 4 Viscosity... 4... 5... 5... 5 Viscosibility...
ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ
 ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΤΜΗΜΑ ΗΛΕΚΤΡΟΛΟΓΩΝ ΜΗΧΑΝΙΚΩΝ & ΜΗΧΑΝΙΚΩΝ ΥΠΟΛΟΓΙΣΤΩΝ ΤΟΜΕΑΣ ΗΛΕΚΤΡΟΝΙΚΗΣ ΜΕΛΕΤΗ ΚΑΙ ΚΑΤΑΣΚΕΥΗ ΙΑΤΑΞΗΣ ΜΕΤΡΗΣΗΣ ΘΕΡΜΟΧΩΡΗΤΙΚΟΤΗΤΑΣ ΣΕ ΜΑΓΝΗΤΙΚΟ ΠΕ ΙΟ ΓΙΑ ΧΑΡΑΚΤΗΡΙΣΜΟ
ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΤΜΗΜΑ ΗΛΕΚΤΡΟΛΟΓΩΝ ΜΗΧΑΝΙΚΩΝ & ΜΗΧΑΝΙΚΩΝ ΥΠΟΛΟΓΙΣΤΩΝ ΤΟΜΕΑΣ ΗΛΕΚΤΡΟΝΙΚΗΣ ΜΕΛΕΤΗ ΚΑΙ ΚΑΤΑΣΚΕΥΗ ΙΑΤΑΞΗΣ ΜΕΤΡΗΣΗΣ ΘΕΡΜΟΧΩΡΗΤΙΚΟΤΗΤΑΣ ΣΕ ΜΑΓΝΗΤΙΚΟ ΠΕ ΙΟ ΓΙΑ ΧΑΡΑΚΤΗΡΙΣΜΟ
Chapter 6: Systems of Linear Differential. be continuous functions on the interval
 Chapter 6: Systems of Linear Differential Equations Let a (t), a 2 (t),..., a nn (t), b (t), b 2 (t),..., b n (t) be continuous functions on the interval I. The system of n first-order differential equations
Chapter 6: Systems of Linear Differential Equations Let a (t), a 2 (t),..., a nn (t), b (t), b 2 (t),..., b n (t) be continuous functions on the interval I. The system of n first-order differential equations
Aluminum Electrolytic Capacitors
 Aluminum Electrolytic Capacitors Snap-In, Mini., 105 C, High Ripple APS TS-NH ECE-S (G) Series: TS-NH Features Long life: 105 C 2,000 hours; high ripple current handling ability Wide CV value range (47
Aluminum Electrolytic Capacitors Snap-In, Mini., 105 C, High Ripple APS TS-NH ECE-S (G) Series: TS-NH Features Long life: 105 C 2,000 hours; high ripple current handling ability Wide CV value range (47
Chapter 7 Transformations of Stress and Strain
 Chapter 7 Transformations of Stress and Strain INTRODUCTION Transformation of Plane Stress Mohr s Circle for Plane Stress Application of Mohr s Circle to 3D Analsis 90 60 60 0 0 50 90 Introduction 7-1
Chapter 7 Transformations of Stress and Strain INTRODUCTION Transformation of Plane Stress Mohr s Circle for Plane Stress Application of Mohr s Circle to 3D Analsis 90 60 60 0 0 50 90 Introduction 7-1
Second Order Partial Differential Equations
 Chapter 7 Second Order Partial Differential Equations 7.1 Introduction A second order linear PDE in two independent variables (x, y Ω can be written as A(x, y u x + B(x, y u xy + C(x, y u u u + D(x, y
Chapter 7 Second Order Partial Differential Equations 7.1 Introduction A second order linear PDE in two independent variables (x, y Ω can be written as A(x, y u x + B(x, y u xy + C(x, y u u u + D(x, y
Ελαφρές κυψελωτές πλάκες - ένα νέο προϊόν για την επιπλοποιία και ξυλουργική. ΒΑΣΙΛΕΙΟΥ ΒΑΣΙΛΕΙΟΣ και ΜΠΑΡΜΠΟΥΤΗΣ ΙΩΑΝΝΗΣ
 Ελαφρές κυψελωτές πλάκες - ένα νέο προϊόν για την επιπλοποιία και ξυλουργική ΒΑΣΙΛΕΙΟΥ ΒΑΣΙΛΕΙΟΣ και ΜΠΑΡΜΠΟΥΤΗΣ ΙΩΑΝΝΗΣ Αριστοτέλειο Πανεπιστήµιο Θεσσαλονίκης Σχολή ασολογίας και Φυσικού Περιβάλλοντος,
Ελαφρές κυψελωτές πλάκες - ένα νέο προϊόν για την επιπλοποιία και ξυλουργική ΒΑΣΙΛΕΙΟΥ ΒΑΣΙΛΕΙΟΣ και ΜΠΑΡΜΠΟΥΤΗΣ ΙΩΑΝΝΗΣ Αριστοτέλειο Πανεπιστήµιο Θεσσαλονίκης Σχολή ασολογίας και Φυσικού Περιβάλλοντος,
Fin coil calculation with NTU
 Fin coil calculation with NTU In all our software applications we never use values like Wi, Pi, Ri, NTUi and ϴ because one don't need it to calculate a heat exchanger like a fin coil. Somebody like the
Fin coil calculation with NTU In all our software applications we never use values like Wi, Pi, Ri, NTUi and ϴ because one don't need it to calculate a heat exchanger like a fin coil. Somebody like the
Comparison of experimental and estimated polarizabilities for organic compounds using ThermoML Archive data
 Comparison of experimental and estimated polarizabilities for organic compounds using ThermoML Archive data Axel Drefahl E-mail: axeleratio@yahoo.com Internet: www.axeleratio.com We compare values for
Comparison of experimental and estimated polarizabilities for organic compounds using ThermoML Archive data Axel Drefahl E-mail: axeleratio@yahoo.com Internet: www.axeleratio.com We compare values for
Enthalpy data for the reacting species are given in the table below. The activation energy decreases when the temperature is increased.
 Q1.This question is about the reaction given below. O(g) + H 2O(g) O 2(g) + H 2(g) Enthalpy data for the reacting species are given in the table below. Substance O(g) H 2O(g) O 2(g) H 2(g) ΔH / kj mol
Q1.This question is about the reaction given below. O(g) + H 2O(g) O 2(g) + H 2(g) Enthalpy data for the reacting species are given in the table below. Substance O(g) H 2O(g) O 2(g) H 2(g) ΔH / kj mol
Απόκριση σε Μοναδιαία Ωστική Δύναμη (Unit Impulse) Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο. Απόστολος Σ.
 Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο The time integral of a force is referred to as impulse, is determined by and is obtained from: Newton s 2 nd Law of motion states that the action
Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο The time integral of a force is referred to as impulse, is determined by and is obtained from: Newton s 2 nd Law of motion states that the action
CHAPTER 48 APPLICATIONS OF MATRICES AND DETERMINANTS
 CHAPTER 48 APPLICATIONS OF MATRICES AND DETERMINANTS EXERCISE 01 Page 545 1. Use matrices to solve: 3x + 4y x + 5y + 7 3x + 4y x + 5y 7 Hence, 3 4 x 0 5 y 7 The inverse of 3 4 5 is: 1 5 4 1 5 4 15 8 3
CHAPTER 48 APPLICATIONS OF MATRICES AND DETERMINANTS EXERCISE 01 Page 545 1. Use matrices to solve: 3x + 4y x + 5y + 7 3x + 4y x + 5y 7 Hence, 3 4 x 0 5 y 7 The inverse of 3 4 5 is: 1 5 4 1 5 4 15 8 3
Thermofluids Data Book for Part I of the Engineering Tripos
 Thermofluids Data Book for Part I of the Engineering Tripos 004 Edition Cambridge University Engineering Department CONTENTS Thermodynamic definitions & relationships... Ideal gas relationships... 3 Perfect
Thermofluids Data Book for Part I of the Engineering Tripos 004 Edition Cambridge University Engineering Department CONTENTS Thermodynamic definitions & relationships... Ideal gas relationships... 3 Perfect
Section 9.2 Polar Equations and Graphs
 180 Section 9. Polar Equations and Graphs In this section, we will be graphing polar equations on a polar grid. In the first few examples, we will write the polar equation in rectangular form to help identify
180 Section 9. Polar Equations and Graphs In this section, we will be graphing polar equations on a polar grid. In the first few examples, we will write the polar equation in rectangular form to help identify
Supplementary Information. Living Ring-Opening Polymerization of Lactones by N-Heterocyclic Olefin/Al(C 6 F 5 ) 3
 Supplementary Information Living Ring-Opening Polymerization of Lactones by N-Heterocyclic Olefin/Al(C 6 F 5 ) 3 Lewis Pairs: Structures of Intermediates, Kinetics, and Mechanism Qianyi Wang, Wuchao Zhao,
Supplementary Information Living Ring-Opening Polymerization of Lactones by N-Heterocyclic Olefin/Al(C 6 F 5 ) 3 Lewis Pairs: Structures of Intermediates, Kinetics, and Mechanism Qianyi Wang, Wuchao Zhao,
Spherical Coordinates
 Spherical Coordinates MATH 311, Calculus III J. Robert Buchanan Department of Mathematics Fall 2011 Spherical Coordinates Another means of locating points in three-dimensional space is known as the spherical
Spherical Coordinates MATH 311, Calculus III J. Robert Buchanan Department of Mathematics Fall 2011 Spherical Coordinates Another means of locating points in three-dimensional space is known as the spherical
Aquinas College. Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET
 Aquinas College Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET Pearson Edexcel Level 3 Advanced Subsidiary and Advanced GCE in Mathematics and Further Mathematics Mathematical
Aquinas College Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET Pearson Edexcel Level 3 Advanced Subsidiary and Advanced GCE in Mathematics and Further Mathematics Mathematical
DERIVATION OF MILES EQUATION FOR AN APPLIED FORCE Revision C
 DERIVATION OF MILES EQUATION FOR AN APPLIED FORCE Revision C By Tom Irvine Email: tomirvine@aol.com August 6, 8 Introduction The obective is to derive a Miles equation which gives the overall response
DERIVATION OF MILES EQUATION FOR AN APPLIED FORCE Revision C By Tom Irvine Email: tomirvine@aol.com August 6, 8 Introduction The obective is to derive a Miles equation which gives the overall response
Section 8.3 Trigonometric Equations
 99 Section 8. Trigonometric Equations Objective 1: Solve Equations Involving One Trigonometric Function. In this section and the next, we will exple how to solving equations involving trigonometric functions.
99 Section 8. Trigonometric Equations Objective 1: Solve Equations Involving One Trigonometric Function. In this section and the next, we will exple how to solving equations involving trigonometric functions.
Supporting Information
 Supporting Information On the Ambiguity of 1,3,2-Benzodiazaboroles as Donor/Acceptor unctionalities in Luminescent Molecules Lothar Weber *[a], Johannes Halama [a], Kenny Hanke [a], Lena Böhling [a], Andreas
Supporting Information On the Ambiguity of 1,3,2-Benzodiazaboroles as Donor/Acceptor unctionalities in Luminescent Molecules Lothar Weber *[a], Johannes Halama [a], Kenny Hanke [a], Lena Böhling [a], Andreas
Inverse trigonometric functions & General Solution of Trigonometric Equations. ------------------ ----------------------------- -----------------
 Inverse trigonometric functions & General Solution of Trigonometric Equations. 1. Sin ( ) = a) b) c) d) Ans b. Solution : Method 1. Ans a: 17 > 1 a) is rejected. w.k.t Sin ( sin ) = d is rejected. If sin
Inverse trigonometric functions & General Solution of Trigonometric Equations. 1. Sin ( ) = a) b) c) d) Ans b. Solution : Method 1. Ans a: 17 > 1 a) is rejected. w.k.t Sin ( sin ) = d is rejected. If sin
Solutions to Exercise Sheet 5
 Solutions to Eercise Sheet 5 jacques@ucsd.edu. Let X and Y be random variables with joint pdf f(, y) = 3y( + y) where and y. Determine each of the following probabilities. Solutions. a. P (X ). b. P (X
Solutions to Eercise Sheet 5 jacques@ucsd.edu. Let X and Y be random variables with joint pdf f(, y) = 3y( + y) where and y. Determine each of the following probabilities. Solutions. a. P (X ). b. P (X
938 Thermodynamics TABLE A 18. Ideal-gas properties of nitrogen, N 2 _ _ T
 cen84959c18-ap01.qxd 8/11/06 1:21 PM Page 938 938 ermodynamics ABLE A 18 Ideal-gas properties of nitrogen, N 2 0 0 0 0 600 17,563 12,574 212.066 220 6,391 4,562 182.639 610 17,864 12,792 212.564 230 6,683
cen84959c18-ap01.qxd 8/11/06 1:21 PM Page 938 938 ermodynamics ABLE A 18 Ideal-gas properties of nitrogen, N 2 0 0 0 0 600 17,563 12,574 212.066 220 6,391 4,562 182.639 610 17,864 12,792 212.564 230 6,683
5.4 The Poisson Distribution.
 The worst thing you can do about a situation is nothing. Sr. O Shea Jackson 5.4 The Poisson Distribution. Description of the Poisson Distribution Discrete probability distribution. The random variable
The worst thing you can do about a situation is nothing. Sr. O Shea Jackson 5.4 The Poisson Distribution. Description of the Poisson Distribution Discrete probability distribution. The random variable
Matrices and Determinants
 Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
DiracDelta. Notations. Primary definition. Specific values. General characteristics. Traditional name. Traditional notation
 DiracDelta Notations Traditional name Dirac delta function Traditional notation x Mathematica StandardForm notation DiracDeltax Primary definition 4.03.02.000.0 x Π lim ε ; x ε0 x 2 2 ε Specific values
DiracDelta Notations Traditional name Dirac delta function Traditional notation x Mathematica StandardForm notation DiracDeltax Primary definition 4.03.02.000.0 x Π lim ε ; x ε0 x 2 2 ε Specific values
6.3 Forecasting ARMA processes
 122 CHAPTER 6. ARMA MODELS 6.3 Forecasting ARMA processes The purpose of forecasting is to predict future values of a TS based on the data collected to the present. In this section we will discuss a linear
122 CHAPTER 6. ARMA MODELS 6.3 Forecasting ARMA processes The purpose of forecasting is to predict future values of a TS based on the data collected to the present. In this section we will discuss a linear
Finite Field Problems: Solutions
 Finite Field Problems: Solutions 1. Let f = x 2 +1 Z 11 [x] and let F = Z 11 [x]/(f), a field. Let Solution: F =11 2 = 121, so F = 121 1 = 120. The possible orders are the divisors of 120. Solution: The
Finite Field Problems: Solutions 1. Let f = x 2 +1 Z 11 [x] and let F = Z 11 [x]/(f), a field. Let Solution: F =11 2 = 121, so F = 121 1 = 120. The possible orders are the divisors of 120. Solution: The
Higher Derivative Gravity Theories
 Higher Derivative Gravity Theories Black Holes in AdS space-times James Mashiyane Supervisor: Prof Kevin Goldstein University of the Witwatersrand Second Mandelstam, 20 January 2018 James Mashiyane WITS)
Higher Derivative Gravity Theories Black Holes in AdS space-times James Mashiyane Supervisor: Prof Kevin Goldstein University of the Witwatersrand Second Mandelstam, 20 January 2018 James Mashiyane WITS)
Paper Reference. Paper Reference(s) 6665/01 Edexcel GCE Core Mathematics C3 Advanced. Thursday 11 June 2009 Morning Time: 1 hour 30 minutes
 Centre No. Candidate No. Paper Reference(s) 6665/01 Edexcel GCE Core Mathematics C3 Advanced Thursday 11 June 2009 Morning Time: 1 hour 30 minutes Materials required for examination Mathematical Formulae
Centre No. Candidate No. Paper Reference(s) 6665/01 Edexcel GCE Core Mathematics C3 Advanced Thursday 11 June 2009 Morning Time: 1 hour 30 minutes Materials required for examination Mathematical Formulae
Fourier Series. MATH 211, Calculus II. J. Robert Buchanan. Spring Department of Mathematics
 Fourier Series MATH 211, Calculus II J. Robert Buchanan Department of Mathematics Spring 2018 Introduction Not all functions can be represented by Taylor series. f (k) (c) A Taylor series f (x) = (x c)
Fourier Series MATH 211, Calculus II J. Robert Buchanan Department of Mathematics Spring 2018 Introduction Not all functions can be represented by Taylor series. f (k) (c) A Taylor series f (x) = (x c)
CENTRIFUGAL AIR COOLED CONDENSERS CONDENSADORES DE AIRE CENTRÍFUGOS. GPC, GMC and GSC Series. Series GPC, GMC y GSC
 CENTRIFUGAL AIR COOLED CONDENSERS GPC, GMC and GSC Series CONDENSADORES DE AIRE CENTRÍFUGOS Series GPC, GMC y GSC Key Example / Ejemplo de nomenclatura de modelos GP Direct Drive 900/100 rpm / Transmisión
CENTRIFUGAL AIR COOLED CONDENSERS GPC, GMC and GSC Series CONDENSADORES DE AIRE CENTRÍFUGOS Series GPC, GMC y GSC Key Example / Ejemplo de nomenclatura de modelos GP Direct Drive 900/100 rpm / Transmisión
MATH423 String Theory Solutions 4. = 0 τ = f(s). (1) dτ ds = dxµ dτ f (s) (2) dτ 2 [f (s)] 2 + dxµ. dτ f (s) (3)
![MATH423 String Theory Solutions 4. = 0 τ = f(s). (1) dτ ds = dxµ dτ f (s) (2) dτ 2 [f (s)] 2 + dxµ. dτ f (s) (3) MATH423 String Theory Solutions 4. = 0 τ = f(s). (1) dτ ds = dxµ dτ f (s) (2) dτ 2 [f (s)] 2 + dxµ. dτ f (s) (3)](/thumbs/81/84712331.jpg) 1. MATH43 String Theory Solutions 4 x = 0 τ = fs). 1) = = f s) ) x = x [f s)] + f s) 3) equation of motion is x = 0 if an only if f s) = 0 i.e. fs) = As + B with A, B constants. i.e. allowe reparametrisations
1. MATH43 String Theory Solutions 4 x = 0 τ = fs). 1) = = f s) ) x = x [f s)] + f s) 3) equation of motion is x = 0 if an only if f s) = 0 i.e. fs) = As + B with A, B constants. i.e. allowe reparametrisations
4 Way Reversing Valve
 STANDARD 4 Way Reversing Valve SHF series four-way reversing valves are applicable for heat pump systems such as central, unitary and room air conditioners to realize switching between cooling mode and
STANDARD 4 Way Reversing Valve SHF series four-way reversing valves are applicable for heat pump systems such as central, unitary and room air conditioners to realize switching between cooling mode and
( ) 2 and compare to M.
 Problems and Solutions for Section 4.2 4.9 through 4.33) 4.9 Calculate the square root of the matrix 3!0 M!0 8 Hint: Let M / 2 a!b ; calculate M / 2!b c ) 2 and compare to M. Solution: Given: 3!0 M!0 8
Problems and Solutions for Section 4.2 4.9 through 4.33) 4.9 Calculate the square root of the matrix 3!0 M!0 8 Hint: Let M / 2 a!b ; calculate M / 2!b c ) 2 and compare to M. Solution: Given: 3!0 M!0 8
 1 1 1 2 1 2 2 1 43 123 5 122 3 1 312 1 1 122 1 1 1 1 6 1 7 1 6 1 7 1 3 4 2 312 43 4 3 3 1 1 4 1 1 52 122 54 124 8 1 3 1 1 1 1 1 152 1 1 1 1 1 1 152 1 5 1 152 152 1 1 3 9 1 159 9 13 4 5 1 122 1 4 122 5
1 1 1 2 1 2 2 1 43 123 5 122 3 1 312 1 1 122 1 1 1 1 6 1 7 1 6 1 7 1 3 4 2 312 43 4 3 3 1 1 4 1 1 52 122 54 124 8 1 3 1 1 1 1 1 152 1 1 1 1 1 1 152 1 5 1 152 152 1 1 3 9 1 159 9 13 4 5 1 122 1 4 122 5
Table of Contents 1 Supplementary Data MCD
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
ANSWERSHEET (TOPIC = DIFFERENTIAL CALCULUS) COLLECTION #2. h 0 h h 0 h h 0 ( ) g k = g 0 + g 1 + g g 2009 =?
 Teko Classes IITJEE/AIEEE Maths by SUHAAG SIR, Bhopal, Ph (0755) 3 00 000 www.tekoclasses.com ANSWERSHEET (TOPIC DIFFERENTIAL CALCULUS) COLLECTION # Question Type A.Single Correct Type Q. (A) Sol least
Teko Classes IITJEE/AIEEE Maths by SUHAAG SIR, Bhopal, Ph (0755) 3 00 000 www.tekoclasses.com ANSWERSHEET (TOPIC DIFFERENTIAL CALCULUS) COLLECTION # Question Type A.Single Correct Type Q. (A) Sol least
forms This gives Remark 1. How to remember the above formulas: Substituting these into the equation we obtain with
 Week 03: C lassification of S econd- Order L inear Equations In last week s lectures we have illustrated how to obtain the general solutions of first order PDEs using the method of characteristics. We
Week 03: C lassification of S econd- Order L inear Equations In last week s lectures we have illustrated how to obtain the general solutions of first order PDEs using the method of characteristics. We
ΠΕΡΙΕΧΟΜΕΝΑ. Μάρκετινγκ Αθλητικών Τουριστικών Προορισμών 1
 ΠΑΝΕΠΙΣΤΗΜΙΟ ΑΙΓΑΙΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΤΗΣ ΔΙΟΙΚΗΣΗΣ ΤΜΗΜΑ ΔΙΟΙΚΗΣΗΣ ΕΠΙΧΕΙΡΗΣΕΩΝ ΔΙΑΤΜΗΜΑΤΙΚΟ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ «Σχεδιασμός, Διοίκηση και Πολιτική του Τουρισμού» ΜΑΡΚΕΤΙΝΓΚ ΑΘΛΗΤΙΚΩΝ ΤΟΥΡΙΣΤΙΚΩΝ
ΠΑΝΕΠΙΣΤΗΜΙΟ ΑΙΓΑΙΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΤΗΣ ΔΙΟΙΚΗΣΗΣ ΤΜΗΜΑ ΔΙΟΙΚΗΣΗΣ ΕΠΙΧΕΙΡΗΣΕΩΝ ΔΙΑΤΜΗΜΑΤΙΚΟ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ «Σχεδιασμός, Διοίκηση και Πολιτική του Τουρισμού» ΜΑΡΚΕΤΙΝΓΚ ΑΘΛΗΤΙΚΩΝ ΤΟΥΡΙΣΤΙΚΩΝ
Calculating the propagation delay of coaxial cable
 Your source for quality GNSS Networking Solutions and Design Services! Page 1 of 5 Calculating the propagation delay of coaxial cable The delay of a cable or velocity factor is determined by the dielectric
Your source for quality GNSS Networking Solutions and Design Services! Page 1 of 5 Calculating the propagation delay of coaxial cable The delay of a cable or velocity factor is determined by the dielectric
( )( ) ( ) ( )( ) ( )( ) β = Chapter 5 Exercise Problems EX α So 49 β 199 EX EX EX5.4 EX5.5. (a)
 hapter 5 xercise Problems X5. α β α 0.980 For α 0.980, β 49 0.980 0.995 For α 0.995, β 99 0.995 So 49 β 99 X5. O 00 O or n 3 O 40.5 β 0 X5.3 6.5 μ A 00 β ( 0)( 6.5 μa) 8 ma 5 ( 8)( 4 ) or.88 P on + 0.0065
hapter 5 xercise Problems X5. α β α 0.980 For α 0.980, β 49 0.980 0.995 For α 0.995, β 99 0.995 So 49 β 99 X5. O 00 O or n 3 O 40.5 β 0 X5.3 6.5 μ A 00 β ( 0)( 6.5 μa) 8 ma 5 ( 8)( 4 ) or.88 P on + 0.0065
Instruction Execution Times
 1 C Execution Times InThisAppendix... Introduction DL330 Execution Times DL330P Execution Times DL340 Execution Times C-2 Execution Times Introduction Data Registers This appendix contains several tables
1 C Execution Times InThisAppendix... Introduction DL330 Execution Times DL330P Execution Times DL340 Execution Times C-2 Execution Times Introduction Data Registers This appendix contains several tables
Partial Differential Equations in Biology The boundary element method. March 26, 2013
 The boundary element method March 26, 203 Introduction and notation The problem: u = f in D R d u = ϕ in Γ D u n = g on Γ N, where D = Γ D Γ N, Γ D Γ N = (possibly, Γ D = [Neumann problem] or Γ N = [Dirichlet
The boundary element method March 26, 203 Introduction and notation The problem: u = f in D R d u = ϕ in Γ D u n = g on Γ N, where D = Γ D Γ N, Γ D Γ N = (possibly, Γ D = [Neumann problem] or Γ N = [Dirichlet
SOLUTIONS TO MATH38181 EXTREME VALUES AND FINANCIAL RISK EXAM
 SOLUTIONS TO MATH38181 EXTREME VALUES AND FINANCIAL RISK EXAM Solutions to Question 1 a) The cumulative distribution function of T conditional on N n is Pr (T t N n) Pr (max (X 1,..., X N ) t N n) Pr (max
SOLUTIONS TO MATH38181 EXTREME VALUES AND FINANCIAL RISK EXAM Solutions to Question 1 a) The cumulative distribution function of T conditional on N n is Pr (T t N n) Pr (max (X 1,..., X N ) t N n) Pr (max
Εργαστήριο Ανάπτυξης Εφαρμογών Βάσεων Δεδομένων. Εξάμηνο 7 ο
 Εργαστήριο Ανάπτυξης Εφαρμογών Βάσεων Δεδομένων Εξάμηνο 7 ο Procedures and Functions Stored procedures and functions are named blocks of code that enable you to group and organize a series of SQL and PL/SQL
Εργαστήριο Ανάπτυξης Εφαρμογών Βάσεων Δεδομένων Εξάμηνο 7 ο Procedures and Functions Stored procedures and functions are named blocks of code that enable you to group and organize a series of SQL and PL/SQL
RECIPROCATING COMPRESSOR CALCULATION SHEET
 Gas properties, flowrate and conditions 1 Gas name Air RECIPRCATING CMPRESSR CALCULATIN SHEET WITH INTERCLER ( Sheet : 1. f 4.) Item or symbol Quantity Unit Item or symbol Quantity Unit 2 Suction pressure,
Gas properties, flowrate and conditions 1 Gas name Air RECIPRCATING CMPRESSR CALCULATIN SHEET WITH INTERCLER ( Sheet : 1. f 4.) Item or symbol Quantity Unit Item or symbol Quantity Unit 2 Suction pressure,
