Figure S1. Characterization of cells and CDK4/6 inhibitors. A, FACS plot for
|
|
|
- Ἑστία Δαγκλής
- 7 χρόνια πριν
- Προβολές:
Transcript
1 Supplementary Figure legends Figure S1. Characterization of cells and CDK4/6 inhibitors. A, FACS plot for PD-1 from wildtype and PD-1-overexpressing Jurkat cells, or CD3+ CD4+ cells from PBMCs from two human donors. B, Kinome binding specificity of palbociclib and abemaciclib at 1nM and 1 nm measured by competition binding assays. CDK4 is indicated in blue. C, IC values of GSK3α/β by CDK4/6 inhibitors. D, Immunoblot for active (non-phosphorylated) or total β-catenin from lysates from PD-1-Jurkat cells treated as indicated for 6h. E, Representative bright-field microscopy images of patient-derived organotypic tumor spheroid (PDOTS) cultured in a three-dimensional culturing system after treatment with different drugs as indicated. Photos were taken one day after the treatment. F, Percentages of subpopulations within total tumor infiltrating leukocytes (% in CD4+ cells). G, Percentage of subpopulations among all live cells, including tumor cells and other stromal cells (% in live cells). Figure S2. CDK6 phosphorylates serine residues of the regulatory domain of NFAT4 (NFATc3). A, 1 H- 1 N-HSQC spectrum of NFATc3 (1-4) alone (red, left panel), overlaid with the spectrum after the addition of recombinant CDK4/cyclin D1 (teal, middle panel), or overlaid with the spectrum after the addition of recombinant CDK6/cyclin D3 (blue, right panel). B, 1 H- 1 N-HSQC spectrum of NFATc3 (red) overlaid with the spectrum of the same protein sample in the presence of.7% DMSO (navy, left panel), overlaid with the spectrum after CDK6- dependent phosphorylation in the presence of.7% DMSO (blue, middle panel), or overlaid with the spectrum in the presence of CDK6 pre-incubated with
2 palbociclib (cyan, right panel). C, Intrinsic disorder prediction of NFATc3 (1-4) using the PONDR-Fit 3, showing that most of the N-terminal regulatory domain of NFATc3 is unstructured, with a few structured elements. Figure S3. Analysis of lung tumor immune infiltrates after CDK4/6 inhibition from Kras G12D (Kras), Kras G12D Lkb1 (KL) or Kras G12D Trp3 fl/fl (KP) mice. Genetically engineered mouse models (GEMMs) harboring Kras, KP or KL mutations were induced to form tumors by Ad-CRE recombinase administration, as verified by MRI scan. Then the mice were treated with either palbociclib or trilaciclib (1 mg/kg, PO) every day for 7 days. A, Percentage of lung-infiltrating T cells within CD4 + total leukocytes after treatment. Left panel, Kras mice treated with trilaciclib. Middle panel, KL mice treated with trilaciclib. Right panel, KL mice treated with palbociclib. (n=3) B, Percentages of Tregs within CD4 + T cells after trilaciclib or palbociclib treatment. (n=3) C. Changes of Treg percentage within CD4 + TILs from KP GEMM mice after palbociclib (upper panel) or trilaciclib (lower panel) treatment. (n=) D, Expression of PD-1 on CD4 + or CD8 + TILs within tumors after trilaciclib or palbociclib treatment. (n=3) E, Expression of CTLA-4 on CD4 + or CD8 + TILs after trilaciclib or palbociclib treatment. (n=3) F, representative flow panels showing PD-1 (left panels) and Ctla-4 (right panels) expression levels from CD4+ T cells after palbociclib treatment. Figure S4. T cell proliferation and cytokine/chemokine profiling of Kras G12D Trp3 fl/fl GEMM mice. A, BrdU incorporation by CD8+ T cells shows proliferation affected by CDK4/6 inhibitors trilaciclib or palbociclib. Mice without (naïve) or with (TMB) Kras LSL-G12D Trp3 fl/fl (KP) allograft tumor were treated with
3 trilaciclib or palbociclib., followed by BrdU injection systemically (I.P.). BrdU incorporation was determined by flow cytometry. (n=6) B, Splenocytes from mice with (TMB) or without (naive) Kras G12D Trp3 fl/fl tumor were dissociated and labeled with CFSE. Cells were cultured and treated with trilaciclib at the indicated concentrations in the presence of α-cd3/cd28 antibody stimulation. After 2 days of stimulation, proliferating cells were quantified as CFSE low cells compared to unstimulated controls for CD4 + (upper panel) and CD8 + (lower panel) T cells. C, BAL fluid was collected 7 days after the treatment of trilaciclib and analyzed for cytokine profiling using Luminex. Expression levels of the analyzed cytokines were expressed as log-2 fold change (L2FC) relative to vehicle control group and shown as a heatmap. Each column represents one mouse. D, Absolute expression levels of IL-6, IL-1, IL-23 and Cxcl9 from control or trilaciclib-treated mice as shown in panel c. Figure S. Tumor antigen experienced T cells are more sensitive to CDK4/6 inhibition. A, Naïve C7BL/6 mice were treated with trilaciclib (1 mg/kg, IP) or vehicle for three days. Two and seven days after the final treatment, splenocytes were isolated and stimulated with α-cd3/cd28 antibodies for 72 hours to measure IFNγ production by ELISA. B, C7BL/6 mice were implanted with B16F1 tumor cells. Seven days post implantation, mice were treated with trilaciclib (1 mg/kg, IP) or vehicle for days. Five days post final treatment, splenocytes were isolated and stimulated with α-cd3/cd28 antibodies for IFNγ production by ELISA. C, IL- 2 production from Tconv (CD4 + CD2 - ) cells after trilaciclib treatment. Tconv cells were isolated from either naïve or tumor bearing (TMB) mice and treated with
4 trilaciclib at indicated concentrations, in the presence of CD3 and CD28 antibodies. IL-2 production was determined 3 days after the treatment as percentage. (n=3) IFNγ production in CD8 + T cells alone from naïve or TMB mice after treatment with trilaciclib, as shown by percentage (D) or fold change (E). (n=3) F, Increased IFNγ production in CD8 + T cells by trilaciclib in the presence of Tregs. CD8 + T cells from naïve or TMB mice were isolated and co-cultured with CD4 + CD2 + regulatory T cells (:1 ratio), in the presence of different concentrations of trilaciclib as indicated. IFNγ production was determined 3 days after the treatment as shown as percentage. (n=3). Figure S6. Short-term CDK4/6 inhibition alters the cell cycle status of tumor infiltrating T cells. A, Bar graph of cell cycle status of T cells, as analyzed by single-cell RNA-seq, from each group of cells with or without trilaciclib treatment, determined by cyclone classification tool. B, 2D t-sne plot with cell cycle stage overlaid to depict the status of each cell. C, The human KRAS-TP3 mutant CLC cell line H38 was treated with CDK4/6 inhibitors (1nM) for 24 hrs. Cell lysate were collected for Western blot showing changes in the levels of phospho- Rb, p-akt and p-erk. D, Changes in cell cycle status of H38 after treatment by CDK4/6 inhibitors in panel C. E, Tumor-infiltrating CD3 + T cells were isolated from lung tumors from KP GEMM mice after 7 days o f treatment with trilaciclib and analyzed by single-cell RNA-seq. Tumor infiltrating T cells were sorted for singlecell RNA-seq analysis. These cells from either trilaciclib treated or control group were identified as three homogeneous groups based on dimension reduction with
5 t-sne combined with density based clustering (dbscan). Violin plot showing NFAT regulated genes expression among all three groups. Figure S7. CDK4/6 inhibition induces changes in the expression of activation and suppression marker genes in tumor-infiltrating T cells. A, Tumorinfiltrating CD3 + T cells were isolated from Kras G12D Trp3 fl/fl GEMM mice treated with trilaciclib for 7 days and analyzed by single-cell RNA-seq. According to gene expression signatures, 2D t-distributed Stochastic Neighbor Embedding (t-sne) plot of RNA-seq gene signatures were overlaid with T cell activation marker genes (A) and suppression marker genes (B) for all three groups of cells from trilaciclibtreated or control mice. Figure S8. Combination treatment of CDK4/6 inhibitor and anti-pd-1 antibody elicits anti-tumor immunity. A, Murine-derived organotypic tumor spheroid (MDOTS) were cultured in 3-D microfluidic system, and cell viability was quantified by staining with Live (AO=green) /Dead (PI=red) at day, day 3 and day 6 after treatment of CDK4/6 inhibitors (1nM) alone or in combination with PD-1 antibody (1 μg/ml) as indicated. Data displayed as absolute cell number. B, Quantification of Live/Dead percentage MDOTS generated from MC38 tumor implanted in Rag1 -/- mice. The result was quantified at day 6 after indicated treatment. Statistical analysis is calculated by comparing the indicated treatment group with control group at day 6. C, Live/Dead analysis of MDOTS cultured in 3-D microfluidic culture and treated with CDK4/6 inhibitors trilaciclib or palbociclib (1nM) with PD-1 antibody (1 µg/ml) without or with neutralization antibodies anti-ifn-γ (1µg/ml) or anti-ccl (1 µg/ml) as indicated 6 days after the
6 treatment. The result is displayed as percentage. Statistical analysis is calculated by comparing the indicated treatment group with control group at day 6. (*p<., **p<.1) Figure S9. Combination treatment of CDK4/6 inhibitor and anti-pd-1 antibody on established tumor. A, Quantification of IL-2 production produced by CT26 tumor infiltrating CD4+ T lymphocytes 2 days after the last treatment (day 32), (*p<., **p<.1, ***p<.1). B. Cytokine production from inguinal lymph nodes of mice with MC38 tumors treated with trilaciclib. At the end of the treatment (day 17), mice were sacrificed and inguinal lymph nodes were isolated for cytokine analysis for IL-2 from CD4 +. C, Survival curve of MC38 murine cancer cells were injected subcutaneously into C7BL/6 mice. The mice were treated with either CDK4/6 inhibitor (Palbociclib, 1 mg/kg) intermittently (3 days on, 4 days off) with or without PD-1 antibody (2 μg/mouse, 3 times a week) starting from day 7. Mice were sacrificed when the tumor volume is above 2mm 3 or the animal has reached protocol study endpoint. D, Individual tumor volume change of each mouse from each treatment group from panel C. Figure S1. Effect of TCR stimulation and CDK4/6 inhibition on phosphorylation of NFkB. Immunoblot for phospho-s36-p6 and total p6 from lysates from PD-1-Jurkat cells treated as indicated for 18h.
7 Supplementary Figure S1
8 Supplementary Figure S2
9 A % in CD Kras * CD4 + CD8 + % in CD * KL CD4 + CD8 + % in CD KL CD4 + CD8 + Palb. C F o x p 3 in C D 4 + (% ) C tr l P a lb. N S B D PD-1 (% ) E Ctla-4 (% ) F Foxp3 in CD4 + (%) KP Kras CD4 + CD8 + KP CD4 + CD8 + PD-1 (%) Ctla-4 (%) Foxp3 in CD4 + (%) Kras KL CD4 + CD8 + Kras CD4 + CD8 + Palb. PD-1 (%) Ctla-4 (%) Foxp3 in CD4 + (%) ** KL CD4 + CD8 + * KL CD4 + CD8 + KL * Palb. PD-1 (% ) Ctla-4 (%) F o x p 3 in C D 4 + (% ) KL Palb. CD4 + CD8 + * KL CD4 + CD8 + Palb. C tr l T r ila. N S Palb. PD-1 Ctla-4 Supplementary Figure S3
10 A B BrdU (%) proliferation (%) Vehicle Palb. naive CD8 TMB naive CD4 + TMB CD4 + nm 1 nm 1 nm 1 nm 1 um 1 um nm C CXCL9 CXCL1 IFNγ IL-12 IL-2 IL-6 IL-1 IL-23 CXCL12 bfgf VEGF TNF-α CCL2 CCL CXCL1 GM-CSF IL-17A IL-1β IL-4 Trilaciclib L2FC 2-2 proliferation (%) nm 1 nm 1 nm 1 um 1 um naive CD8 + TMB CD8 + D IL-6 (pg/ml) IL-1 (pg/ml) IL-23 (pg/ml) Cxcl9/MIG (pg/ml) Supplementary Figure S4
11 A 4 3 Vehicle G1T28 Naive IFNg B 1 TMB IFNg Vehicle * G1T28 pg/ml 2 pg/ml 1 D2 unstim D2 stim D7 unstim D7 stim unstim stim C IL-2 (%) naive Tconv TMB Tconv * * D IFNγ (%) naive CD8 + TMB CD E IFNγ (fold) Trilaciclib (nm) naive CD8 + TMB CD8 + F IFNγ (%) Trilaciclib (nm) naive CD8 + +Treg TMB CD8 + +Treg * * Trilaciclib (nm) Trilaciclib (nm) Supplementary Figure S
12 A group_1 group_2 group_3 B 1 7 count dbscan clusters G1 G2M S 2 G1T28 vehicle G1T28 vehicle G1T28 vehicle plate_treatment C DMSO Palb. D prb (S87/811) Total Rb perk1/2 ERK1/2 pakt P e r c e n t ( % ) G 2 S G 1 AKT Actin O b 8 E Supplementary Figure S6
13 A Tnfrsf9 Icosl Tnfrsf Ts2 Ts2 colour 1% 2% 3% % 6% 8% Ts2 plate treatment groups group_1 group_2 group_3 plate treatment groups group_1 group_2 group_3 colour 1% 2% 3% % 6% 8% 1 Ts1 1 Ts1 1 Ts1 1 Cd4 1 Cd8 1 Cd86 Icosl Ts2 Ts2 Ts2 1 Ts2 plate treatment groups group_1 group_2 colour group_3 1% colour 1% 2% 3% % 6% 8% 8% 3% % 6% plate treatment groups 2% group_1 group_2 group_3 B 1 Ts1 1 Ts1 1 Ts1 1 Ts1 Cd274 Pdcd1lg2 Havcr Ts2 plate treatment groups group_1 group_2 group_3 colour Ts2 1% 3% % 6% 8% 2% plate treatment groups group_1 group_2 group_3 colour 1% 3% % 6% 8% 2% Ts2 plate grou grou grou colou 1 3% % 6% 8% 2% 1 Ts1 1 Ts1 1 Ts1 Supplementary Figure S7
14 A Dead Live MC38 MDOTS (cell number) B mean cell area + SD % cells day DMSO+IgG Rag1 MC38 MDOTS Day 3 Day 6 palb. PD1 trila.+pd1 palb.+pd1 Palb. +PD-1 Palb.+PD-1 DMSO+IgG Palb. +PD-1 Palb.+PD-1 Dead Live C % cells 1 1 Dead Live * * MC38 MDOTS * * trila.+pd1 trila.+pd1+αifnγ trila.+pd1+αccl palb.+pd1 palb.+pd1+αifnγ palb.+pd1+αccl * Supplementary Figure S8
15 A IL-2 (%) 1 1 * * Vehicle Palb. PD-1 Palb.+PD-1 B IL-2 (%) Vehicle Trila PD-1 +PD-1 C MC38 1 vehicle S u r v iv a l ( % ) PD-1 Palb. Palb.+PD-1 D W e e k s vehicle Palb. PD-1 Palb.+PD-1 T u m o r V o lu m e ( m m 3 ) D a y s (p o s t im p la n ta tio n ) T u m o r V o lu m e ( m m 3 ) D a y s (p o s t im p la n ta tio n ) T u m o r V o lu m e ( m m 3 ) D a y s (p o s t im p la n ta tio n ) T u m o r V o lu m e ( m m 3 ) D a y s (p o s t im p la n ta tio n ) Supplementary Figure S9
16 A Supplementary Figure S1
17 Supplementary Table S1 Rank Vendor ID Chemical_Name Zscore_1 Zscore_2 Zscore_AVG Smiles Canonical_Smiles InChI Mol_Formula Mol_Weight Mol_Mass PubChem_CID SID GSK-3 Inhibitor X c1cc(\c(=n\oc(=o)c)\c(=c(/c(ccc(br)c2)c2n3)\c3=o)\n4)c4cc CC(=O)O\N=C\1/C(=C\2/C(=O)Nc3cc(Br)ccc23)/Nc4ccccc14 InChI=1/C18H12BrN3O3/c1-9(23) C18H12BrN3O (12)2-17(16) (19)8-14(11)21-18(1)24/h ,2H,1H3,(H,21,24)/b17-1-, GSK-3 Inhibitor IX c1cc(\c(=n\o)\c(=c(/c(ccc(br)c2)c2n3)\c3=o)\n4)c4cc1 O\N=C\1/C(=C\2/C(=O)Nc3cc(Br)ccc23)/Nc4ccccc14 InChI=1/C16H1BrN3O2/c (7-8)19-16(21)13(9)1- C16H1BrN3O (2-22) (1)18-1/h1-7,18,22H,(H,19,21)/b ,2-14- Indirubin Derivative E84 c1cc(\c(=n/occc(o)co)\c(=c(\c(=o)n2)/c(cccc3)c23)\n4)c4c OCC(O)CCO\N=C/1\C(=C/2\C(=O)Nc3ccccc23)\Nc4ccccc14 InChI=1/C2H19N3O4/c (2) C2H19N3O c1 16(14)21-19(18) (13)22-2(17)26/h ,12,21,24-2H,9-11H2,(H,22,26)/b19-17-, SB 222 n1c(n)nc(c2n(c3ccncc3)cnc2c4ccc(f)cc4)cc1 Nc1nccc(n1)c2c(ncn2C3CCNCC3)c4ccc(F)cc4 InChI=1/C18H19FN6/c (2-4-13)16-17( C18H19FN (2)24-1)2( ) /h1-4,7, ,14,21H,-6,8-9H2,(H2,2,22,24) Indirubin-3'-monoxime, -Iodo- c1c(\c(=n/o)\c(=c(/c(cc(i)cc2)c2n3)\c3=o)\n4)c4ccc1 O\N=C/1\C(=C/2\C(=O)Nc3ccc(I)cc23)\Nc4ccccc14 InChI=1/C16H1IN3O2/c (7-8)13(16(21)19- C16H1IN3O )1-14(2-22) (9)18-1/h ,18,22H,(H,19,21)/b1-13-,2-14+ KT72 c1(cccc2)c2n([c@]([c@@](c(occcccc)=o)(o)c3)(c)o[c@ CCCCCCOC(=O)[C@]1(O)C[C@@H]2O[C@@]1(C)n3c4ccccc InChI=1/C32H31N3O/c (37)32(38) C32H31N3O cc6CNC(=O)c6c7c8ccccc8n2c7c (21)2-26-2( (26)36) (19)3(28(24)27(2)34)31(32,2)4-23/h6-9,11-14,23,38H,3-,1,1-17H2,1-2H3,(H,33,36)/t ,31+,32+/m/s1 Cdk4 Inhibitor c1(c(c(=o)nc2=o)c2c(c(cccc3)c3[nh]4)c4)c[nh]c(cc(br)cc6) Brc1ccc2c(c1)[nH]c3c4[nH]ccccccc4c6C(=O)NC(=O)c6c23 InChI=1/C2H1BrN3O2/c (7-8) (1)16- C2H1BrN3O c16 1(19(2)24-2(16)26) (9)22-17(13)18/h1-7, H,(H,24,2,26) Akt Inhibitor XII, Isozyme- c1(nc(c2ccc(cn3ccc(n4c(=o)nc(cccc)c4)cc3)cc2)c(c6ccc CN1CCN(CC1)C(=O)c2ccc3nc(c4ccc(CNCCC(CC)N6C(=O)N C78H8N14O4R# Selective, Akti-2 cc6)n7)c7cc(c(=o)n8ccn(c)cc8)cc1.c9(nc(c%1ccc(cn%11 c7ccccc67)cc4)c(nc3c2)c8ccccc8.cn9ccn(cc9)c(=o)c%1cc CCC(N%12C(=O)Nc(cccc%13)c%12%13)CC%11)cc%1)c(c% c%11nc(c%12ccccc%12)c(nc%11c%1)c%13ccc(cn%14ccc( 14ccccc%14)n%1)c%1ccc(C(=O)N%16CCN(C)CC%16)c9.[R CC%14)N%1C(=O)Nc%16ccccc%1%16)cc%13.[RH].[RH] H].[RH] SU916 c1(oc)cc(\c(=c\c2c[nh]cn2)\c(=o)n3)c3cc1 COc1ccc2NC(=O)\C(=C/c3c[nH]cn3)\c2c1 InChI=1/C13H11N3O2/c (-9)11(13(17)16- C13H11N3O ) /h2-7H,1H3,(H,14,1)(H,16,17)/b11-4- JAK3 Inhibitor VI c1ncc(c2cc(\c(=c\c3[nh]ccc3)\c(=o)n4)c4cc2)cc1.cs(o)(=o)= CS(=O)(=O)O.O=C1Nc2ccc(cc2/C/1=C/c3ccc[nH]3)c4cccnc4 InChI=1/C18H13N3O.CH4O3S/c ( C19H17N3O4S O 14)1-9-12(-6-17(1)21-18) ;1-(2,3)4/h ,2H,(H,21,22);1H3,(H,2,3,4)/b16-1-; Compound 41 C1=CN(C(=O)C=C2N3CCOCC3)C(=N2)c(cccc4)c14 O=C1C=C(N=C2N1C=Cc3ccccc23)N4CCOCC4 InChI=1/C16H1N3O2/c ( ) C16H1N3O (13)-6-19(1)16/h1-6,11H,7-1H2 Aurora Kinase Inhibitor III c1cnc(nc2cc(nc(=o)c3cc3)ccc2)nc1nc4cccc(c(f)(f)f)c4 FC(F)(F)c1cccc(Nc2ccnc(Nc3cccc(NC(=O)C4CC4)c3)n2)c1 InChI=1/C21H18F3NO/c22-21(23,24) (11-14)26- C21H18F3NO (29-18) (12-17)27-19(3) /h1-6,9-13H,7-8H2,(H,27,3)(H2,2,26,28,29) DNA-PK Inhibitor III c1(n2ccocc2)ccc(c(c)=o)c(o)c1 CC(=O)c1ccc(cc1O)N2CCOCC2 InChI=1/C12H1NO3/c1-9(14) (8-12(11)1) C12H1NO /h2-3,8,1H,4-7H2,1H3 LY 2942, 4-NH2 c1cc(c(=o)c=c(n2ccocc2)o3)c3c(c4ccc(n)cc4)c1 Nc1ccc(cc1)c2cccc3C(=O)C=C(Oc23)N4CCOCC4 InChI=1/C19H18N2O3/c (-7-14) C19H18N2O (22)12-18(24-19(1)16) /h1-7,12H, ,2H2 GSK-3b Inhibitor XI c1(c2c(nccc2)n(ccco)c1)c(c(=o)nc3=o)=c3c4cnccn4 OCCCn1cc(C2=C(C(=O)NC2=O)c3cnccn3)c4cccnc14 InChI=1/C18H1NO3/c ( C18H1NO (11)23)14-1(18(26)22-17(14)2) /h1,3-6, ,24H,2,7-8H2,(H,22,2,26) Arcyriaflavin A, Synthetic c1(c(c(cccc2)c2[nh]3)c3c([nh]4)cc6c4cccc6)cc(=o)nc1=o O=C1NC(=O)c2c1c3c4ccccc4[nH]c3c[nH]c6ccccc6c2 InChI=1/C2H11N3O2/c (9)21- C2H11N3O (13)18-14(16(1)2(2)23-19) (1)22-18/h ,21-22H,(H,23,24,2) Herbimycin A, Streptomyces C(C1=O)=C2C(=O)C(NC(\C(=C\C=C/C(OC)[C@@H](OC(N)=O) CO[C@H]1C[C@H](C)[C@@H](OC)C2=CC(=O)C=C(NC(=O)\C InChI=1/C3H42N2O9/c (37-)28(41- C3H42N2O sp. \C(\C)=C\[C@H](C)C(OC)[C@@H](OC)C[C@H](C)[C@H]2OC)\ (=C\C=C/C(OC)[C@@H](OC(=O)N)\C(=C\[C@H](C)C1OC)\C)\ 3(31)36)18(3)12-17(2)27(4-8)24(38-6)13-19(4)26(39-7) C)=O)=C1 C)C2=O 2(33)1-22(2(21)34)32-29(16)3/h9-12,14-1,17,19,23-24,26-28H,13H2,1-8H3,(H2,31,36)(H,32,3)/b11-9-,16-1+, /t17-,19-,23?,24-,26+,27?,28-/m/s1 Akt Inhibitor X c1cc(oc(ccc(cl)c2)c2n3ccccn(cc)cc)c3cc1.cl Cl.CCN(CC)CCCCN1c2ccccc2Oc3ccc(Cl)cc13 InChI=1/C2H2ClN2O.ClH/c1-3-22(4-2) C2H26Cl2N2O (17) (21)1-18(2)23;/h-6,9-12,1H,3-4, ,13-14H2,1-2H3;1H Met Kinase Inhibitor c1(s(=o)(=o)n(c2cccc(cl)c2)c)cc(\c(=c\c3c(c)c(c(=o)n4ccn CN(c1cccc(Cl)c1)S(=O)(=O)c2ccc3NC(=O)\C(=C/c4[nH]c(C)c(C InChI=1/C28H3ClNO4S/c1-17-2(3-18(2)26(17)28(36)34- C28H3ClNO4S (C)CC4)c(C)[nH]3)\C(=O)N)ccc1 (=O)NCCN(C)CC)c4C)\c3c (3) ) (8-9-24(22)31-27(23)3)39(37,38)33(4) (29)14-2/h-9, ,3H,1-13H2,1-4H3,(H,31,3)/b Akt Inhibitor V, Triciribine C1(CO)OC(n(c2)c3c(c(ncn3)N(C)N=C4N)c24)C(O)C1O CN1N=C(N)c2cn(C3OC(CO)C(O)C3O)c4ncnc1c24 InChI=1/C13H16N6O4/c (1(14)17-18)2-19(12(7)16- C13H16N6O )13-9(22)8(21)6(3-2)23-13/h2,4,6,8-9,13, H,3H2,1H3,(H2,14,17) Bcr-abl Inhibitor c1(oc(f)(f)f)ccc(nc2ncnc(c3cccc(c(n)=o)c3)c2)cc1 NC(=O)c1cccc(c1)c2cc(Nc3ccc(OC(F)(F)F)cc3)ncn2 InChI=1/C18H13F3N4O2/c19-18(2,21) (-7- C18H13F3N4O ) ( ) (8-11)17(22)26/h H,(H2,22,26)(H,23,24,2) Src Kinase Inhibitor I c1(oc)cc(c(nc2ccc(oc3ccccc3)cc2)ncn4)c4cc1oc COc1cc2ncnc(Nc3ccc(Oc4ccccc4)cc3)c2cc1OC InChI=1/C22H19N3O3/c (13-21(2)27-2)23- C22H19N3O (18) (11-9-1) /h H,1-2H3,(H,23,24,2) IKK-3 Inhibitor IX c1c(occ2c(s(c)(=o)=o)cccc2)c(c#n)sc1n3cnc(cc(oc)c(oc)c COc1cc2ncn(c3cc(OCc4ccccc4S(=O)(=O)C)c(C#N)s3)c2cc1O InChI=1/C22H19N3OS2/c (9-18(17)29- C22H19N3OS )c34 C 2)2( ) (2(11-23)31-22) (14)32(3,26)27/h4-1,13H,12H2,1-3H3 PIKfyve Inhibitor c1(c(n2ccocc2)nc(c3cc(nc(=o)c4cnc(n)cc4)ccc3)n)cc(ccc Nc1ccc(cn1)C(=O)Nc2cccc(c2)c3nc(N4CCOCC4)coc6ncccc6c InChI=1/C2H21N7O3/c ( )24(33) C2H21N7O n6)c6o1 n (13-17) (18)3-21(2)23(31-22) /h1-8,13-14H, H2,(H2,26,28)(H,29,33) Isogranulatimide c1(c(c(=o)nc2=o)c2c(cnc3)n34)c4[nh]c(cccc)c1 O=C1NC(=O)c2c1c3c4ccccc4[nH]c3ncncc2 InChI=1/C1H8N4O2/c (9)13- C1H8N4O (12(11)1(21)18-14) (7)17-13/h ,17H,(H,18,2,21) ATM Kinase Inhibitor C(c1c(Sc(cccc2)c2S3)c3ccc1)(OC(N4CCOCC4)=CC=O)=C O=C1C=C(OC(=C1)c2cccc3Sc4ccccc4Sc23)NCCOCC InChI=1/C21H17NO3S2/c (2-2(13-14) C21H17NO3S ) (1) (18)26-19/h1-7, H,8-11H2 PI 3-Kβ Inhibitor VI, TGX- C1(C)=CN(C(=O)C=C2N3CCOCC3)C(=N2)C(C(C)Nc4ccccc4)= CC(Nc1ccccc1)C2=CC(=CN3C(=O)C=C(N=C23)N4CCOCC4)C InChI=1/C21H24N4O2/c (16(2) C21H24N4O C1 17) (13-2(26)2(21)14-1) /h ,12-14,16,22H,8-11H2,1-2H3 PDGF Receptor Tyrosine c1(oc)cc(c(n2ccn(c(=o)nc3ccc(oc4ccccc4)cc3)cc2)ncn)c COc1cc2ncnc(N3CCN(CC3)C(=O)Nc4ccc(Occcccc)cc4)c2cc InChI=1/C27H27NO4/c (17-2(24)3-2)28- C27H27NO Kinase Inhibitor III cc1oc 1OC (22) ( )27(33) ( ) /h3-11,16-18H,12-1H2,1-2H3,(H,3,33) Chk2 Inhibitor II c1(c2ccc(oc3ccc(cl)cc3)cc2)[nh]c(ccc4c(n)=o)c(c4)n1 NC(=O)c1ccc2[nH]c(nc2c1)c3ccc(Oc4ccc(Cl)cc4)cc3 InChI=1/C2H14ClN3O2/c (9--14) (2- C2H14ClN3O ) (19(22)2)11-18(17)24-2/h H,(H2,22,2)(H,23,24) Flt-3 Inhibitor III c1cc(c2cnc(nc3ccc(occn4cccc4)cc3)s2)ccc1 C(CN1CCCC1)Oc2ccc(Nc3ncc(s3)c4ccccc4)cc2 InChI=1/C21H23N3OS/c (7-3-1) (26-2)23- C21H23N3OS ( ) /h1-3,6-11,16H, ,12-1H2,(H,22,23) EGFR/ErbB-2 Inhibitor c1(oc)cc(c(nc2ccc(occ3ccccc3)cc2)ncn4)c4cc1oc COc1cc2ncnc(Nc3ccc(OCc4ccccc4)cc3)c2cc1OC InChI=1/C23H21N3O3/c (13-22(21)28-2)24- C23H21N3O (19) ( ) /h ,1H,14H2,1-2H3,(H,24,2,26) Indirubin-3'-monoxime c1cc(\c(=n/o)\c(=c(\c2=o)/c3c(n2)cccc3)\n4)c4cc1 O\N=C/1\C(=C/2\C(=O)Nc3ccccc23)\Nc4ccccc14 InChI=1/C16H11N3O2/c ( (9)18-16)1- C16H11N3O (19-21) (1)17-1/h1-8,17,21H,(H,18,2)/b , Flt-3 Inhibitor II c1(o)cc(cc(c(=o)c2cc(cc(o)cc3)c3[nh]2)[nh]4)c4cc1 Oc1ccc2[nH]c(cc2c1)C(=O)c3cc4cc(O)ccc4[nH]3 InChI=1/C17H12N2O3/c (-11)7-1(18- C17H12N2O )17(22) (21)2-4-14(1)19-16/h1-8,18-21H Flt-3 Inhibitor C1Cc(sc(NC(c2cc(OC)c(OC)cc2)=O)c3C(=O)N)c3CC1 COc1ccc(cc1OC)C(=O)Nc2sc3CCCCc3c2C(=O)N InChI=1/C18H2N2O4S/c (9-13(12)24- C18H2N2O4S )17(22)2-18-1(16(19)21) (11)2-18/h7-9H, H2,1-2H3,(H2,19,21)(H,2,22) EGFR Inhibitor n1cnc(nc2cc(nc(=o)c3cc3)ccc2)cc1nc4cccc(c(f)(f)f)c4 FC(F)(F)c1cccc(Nc2cc(Nc3cccc(NC(=O)C4CC4)c3)ncn2)c1 InChI=1/C21H18F3NO/c22-21(23,24) (9-14) C21H18F3NO ( ) (1-16)29-2(3) /h1-6,9-13H,7-8H2,(H,29,3)(H2,2,26,27,28) ERK Inhibitor III c1ccc(c2oc(\c=n/n\c(\n)=n\[n+]([o-])=o)cc2)cc1[n+]([o-])=o N\C(=N/[N+](=O)[O-])\N\N=C/c1oc(cc1)c2cccc(c2)[N+](=O)[O-] InChI=1/C12H1N6O/c13-12(16-18(21)22) C12H1N6O (23-1) (6-8)17(19)2/h1-7H,(H3,13,1,16)/b14-7- Keratinocyte Differentiation c1cc(c(cc(o)=o)c(nc(=o)c(cccc2)c23)n34)c4cc1 OC(=O)Cc1c2NC(=O)c3ccccc3n2c4ccccc14 InChI=1/C17H12N2O3/c2-1(21) (1) C17H12N2O Inducer (14)17(22)18-16(12)19/h H,9H2,(H,18,22)(H,2,21)
18 JNK Inhibitor V PI 3-Kg Inhibitor SU ML-7, Hydrochloride GSK-3 Inhibitor IX, Control, MeBIO Akt Inhibitor VIII, Isozyme- Selective, Akti-1/ BPIQ-I Casein Kinase II Inhibitor III, TBCA Olomoucine II Aurora Kinase Inhibitor II Azakenpaullone SB 238, Sulfone PI 3-KbInhibitor II GTP PI 3-Kα Inhibitor IV Aurora Kinase Inhibitor III p38 MAP Kinase Inhibitor VII, SD Cdk2/ Inhibitor GSK-3b Inhibitor VIII Chk2 Inhibitor SU RSK Inhibitor, SL PD Casein Kinase II Inhibitor I PP JNK Inhibitor, Negative Control JAK3 Inhibitor IV JNK Inhibitor II AGL Adenosine Kinase Inhibitor SB KN IP3K Inhibitor AG cfms Receptor Tyrosine Kinase Inhibitor EGFR/ErbB-2/ErbB-4 Inhibitor Cdk1 Inhibitor, CGP7414A JNK Inhibitor IX Bisindolylmaleimide V PD LY Chelerythrine Chloride c1cc(n\c(=c(\c#n)/c2nc(nccc3cccnc3)ncc2)\s4)c4cc1 N#C\C(=C/1\Nc2ccccc2S1)\c3ccnc(NCCc4cccnc4)n3 InChI=1/C2H16N6S/c ( (17)27- C2H16N6S ) (26-16) /h1-6,8-9,11,13,2H,7,1H2,(H,23,24,26)/b19-1+ c1nc(cc(\c=c(/sc(=o)n2)\c2=o)cc3)c3nc1 O=C1NC(=O)\C(=C\c2ccc3nccnc3c2)\S1 InChI=1/C12H7N3O2S/c (18-12(17)1-11) C12H7N3O2S (-7) /h1-6H,(H,1,16,17)/b1-6- c1c(\c(=c\c2cc(cccc3)c3[nh]2)\c(=o)n4)c4ccc1s(n(c)c)(=o CN(C)S(=O)(=O)c1ccc2NC(=O)\C(=C/c3cc4CCCCc4[nH]3)\c2c InChI=1/C19H21N3O3S/c1-22(2)26(24,2) (11- C19H21N3O3S )=O 1 14)16(19(23)21-18) (12)2-13/h7-11,2H,3-6H2,1-2H3,(H,21,23)/b16-1- c1cc(c(s(=o)(=o)n2ccnccc2)ccc3)c3c(i)c1.[h]cl Cl.Ic1cccc2c(cccc12)S(=O)(=O)N3CCCNCC3 InChI=1/C1H17IN2O2S.ClH/c (14) C1H18ClIN2O2S (13)21(19,2) ;/h1-2,4-7,17H,3,8-11H2;1H c1cc(\c(=n\o)\c(=c(/c(ccc(br)c2)c2n3c)\c3=o)\n4)c4cc1 CN1C(=O)\C(=C/2\C(\c3c(N2)cccc3)=N/O)\c4ccc(Br)cc14 InChI=1/C17H12BrN3O2/c (18)6-7- C17H12BrN3O (13)14(17(21)22)16-1(2-23) (1)19-16/h2-8,19,23H,1H3/b16-14-,2-1- c1(nc(c2ccc(cn3ccc(n4c(=o)nc(cccc)c4)cc3)cc2)c(c6ccc O=C1Nc2ccccc2N1C3CCN(Cc4ccc(cc4)cnc6cc7nc[nH]c7cc6n InChI=1/C34H29N7O/c (26)41(34)2-14- C34H29N7O cc6)n7)c7cc([nh]cn8)c8c1 cc8ccccc8)cc3 16-4(17-1-2) ( )33-32( ) ( )19-3(29)38-33/h1-13,18-19,21,2H,14-17,2H2,(H,3,36)(H,39,42) c1(cc(c(nc2cccc(br)c2)ncn3)c3c4)c4n(c)cn1 Cn1cnc2cc3c(Nc4cccc(Br)c4)ncnc3cc12 InChI=1/C16H12BrN/c (7-1(14)22)18-8- C16H12BrN (12) (17)-11/h2-9H,1H3,(H,18,19,21) c1(br)c(br)c(\c=c\c(=o)o)cc(br)c1br OC(=O)\C=C\c1cc(Br)c(Br)c(Br)c1Br InChI=1/C9H4Br4O2/c1--3-4(1-2- C9H4Br4O (14)1)7(11)9(13)8()12/h1-3H,(H,14,1)/b2-1+ n1c(ncc2ccccc2o)c(ncn3c(c)c)c3nc1n[c@@]([h])(co)cc CC[C@H](CO)Nc1nc(NCc2ccccc2O)c3ncn(C(C)C)c3n1 InChI=1/C19H26N6O2/c1-4-14(1-26) ( C19H26N6O (13)27)16-18(24-19)2( )12(2)3/h-8,11-12,14,26-27H,4,9-1H2,1-3H3,(H2,2,22,23,24)/t14-/m1/s1 c1(oc)cc(c(nc2ccc(nc(c3ccccc3)=o)cc2)ncn4)c4cc1oc COc1cc2ncnc(Nc3ccc(NC(=O)c4ccccc4)cc3)c2cc1OC InChI=1/C23H2N4O3/c (13-21(2)3-2)24- C23H2N4O (18) ( )27-23(28) /h3-14H,1-2H3,(H,27,28)(H,24,2,26) c1cc(nc(=o)cc(c(cc(br)cc2)c2[nh]3)c34)c4nc1 Brc1ccc2[nH]c3c(CC(=O)Nc4cccnc34)c2c1 InChI=1/C1H1BrN3O/c (6-8)1-7-13(2) C1H1BrN3O (12)14(1)19-11/h1-6,19H,7H2,(H,18,2) c1(c2ccncc2)[nh]c(c3ccc(s(c)(=o)=o)cc3)nc1c4ccc(f)cc4 CS(=O)(=O)c1ccc(cc1)c2nc(c3ccc(F)cc3)c([nH]2)c4ccncc4 InChI=1/C21H16FN3O2S/c1-28(26,27) (-9-18) C21H16FN3O2S ( (22)7-3-14)2(2-21) /h2-13H,1H3,(H,24,2) c1(oc(f)(f)o2)c2cc(\c=c(/sc(=o)n3)\c3=o)cc1 FC1(F)Oc2ccc(\C=C\3/SC(=O)NC3=O)cc2O1 InChI=1/C11HF2NO4S/c12-11(13) (3-7(6)18-11)4-8- C11HF2NO4S (1)14-1(16)19-8/h1-4H,(H,14,1,16)/b8-4- c1cc2c(c3c(o2)c(c4ccccc4)n[nh]3)cc1 o1c2ccccc2c3[nh]nc(c4ccccc4)c13 InChI=1/C1H1N2O/c (7-3-1) ( )11-8- C1H1N2O (11)18-1/h1-9H,(H,16,17) c1(c(n2ccocc2)nc(c3cc(o)ccc3)n4)c4ccs1.[h]cl.[h]cl Cl.Cl.Oc1cccc(c1)c2nc(N3CCOCC3)c4sccc4n2 InChI=1/C16H1N3O2S.2ClH/c (1-12) C16H17Cl2N3O2S (13)16(18-1) ;;/h1-4,9-1,2H,- 8H2;2*1H c1cnc(nc2cc(nc(=o)c3cc3)ccc2)nc1nc4cccc(c(f)(f)f)c4 FC(F)(F)c1cccc(Nc2ccnc(Nc3cccc(NC(=O)C4CC4)c3)n2)c1 InChI=1/C21H18F3NO/c22-21(23,24) (11-14)26- C21H18F3NO (29-18) (12-17)27-19(3) /h1-6,9-13H,7-8H2,(H,27,3)(H2,2,26,28,29) c1(c(=o)n)cc(cc[nh]2)c2cc1 NC(=O)c1ccc2[nH]ccc2c1 InChI=1/C9H8N2O/c1-9(12) (-7) /h1- C9H8N2O ,11H,(H2,1,12) c1nc(nc2ccc(s(n)(=o)=o)cc2)cc(n)n1.cl Cl.Nc1cc(Nc2ccc(cc2)S(=O)(=O)N)ncn1 InChI=1/C1H11NO2S.ClH/c ( ) C1H12ClNO2S (4-2-7)18(12,16)17;/h1-6H,(H2,12,16,17)(H3,11,13,14,1);1H C(=O)(NCc1ccc(OC)cc1)Nc2sc([N+]([O-])=O)cn2 COc1ccc(CNC(=O)Nc2ncc(s2)[N+](=O)[O-])cc1 InChI=1/C12H12N4O4S/c (3--9) (17)1-12- C12H12N4O4S (21-12)16(18)19/h2-,7H,6H2,1H3,(H2,13,14,1,17) c1(\c(=c(/n=c(n)n2)\c2=o)\ccnc3=o)c3[nh]c(cccc4)c14.[h] Cl.NC1=N\C(=C/2\CCNC(=O)c3[nH]c4ccccc4c23)\C(=O)N1 InChI=1/C1H13NO2.ClH/c (14(22)2-1) C1H14ClNO Cl 13(21)12-1(8) (7)18-12;/h1-4,18H,- 6H2,(H,17,21)(H3,16,19,2,22);1H/b11-8-; c1(\c(=c\c2c(c)c(c(nccn(cc)cc)=o)c(c)[nh]2)\c(=o)n3)c3c CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C\2/C(=O)Nc3ccc(Cl)cc23) InChI=1/C22H27ClN4O2/c1--27(6-2) (29)2- C22H27ClN4O cc(cl)c1 c1c 13(3)19(2-14(2)4) (23)7-8-18(16)26-21(17)28/h7-8,11-12,2H,-6,9-1H2,1-4H3,(H,24,29)(H,26,28)/b c1(o)cc(oc(c2ccc(o)cc2)=c(oc(oc3c)c(o)c(oc(=o)c)c3o CC1OC(OC2=C(Oc3cc(O)cc(O)c3C2=O)c4ccc(O)cc4)C(O)C(O InChI=1/C2H24O12/c1-1-21(34-11(2)26)24(3- C2H24O C(=O)C)C4=O)c4c(O)c1 C(=O)C)C1OC(=O)C 12(3)27)2(32)2(33-1) (31)18-16(3)8-1(29)9-17(18)36-22(23) (28)7--13/h4-1,2-21,24-2,28-3,32H,1-3H3 c1(nc)cc(c(nc2cccc(br)c2)ncn3)c3cn1 CNc1cc2c(Nc3cccc(Br)c3)ncnc2cn1 InChI=1/C14H12BrN/c ( ) C14H12BrN (11) (1)-1/h2-8H,1H3,(H,16,17)(H,18,19,2) c1(br)c(br)c(nn[nh]2)c2c(br)c1br Brc1c(Br)c(Br)c2[nH]nnc2c1Br InChI=1/C6HBr4N3/c7-1-2(8)4(1)6-(3(1)9) C6HBr4N /h(H,11,12,13) c1(c(n)ncn2)c2n(c3ccccc3)nc1 Nc1ncnc2c1cnn2c3ccccc3 InChI=1/C11H9N/c (11(9) ) C11H9N /h1-7H,(H2,12,13,14) c1c(c(nn2c)c(c2ccc3)c3c4=o)c4ccc1 Cn1nc2c3ccccc3C(=O)c4cccc1c24 InChI=1/C1H1N2O/c (12)14(16-17)9--2- C1H1N2O (9)1(11)18/h2-8H,1H3 c1cc(cc(c(=o)ccn(c(c)c)cc2ccccc2)cc3)c3cc1.cl Cl.CC(C)N(CCC(=O)c1ccc2ccccc2c1)Cc3ccccc3 InChI=1/C23H2NO.ClH/c1-18(2)24( )1-14- C23H26ClNO (2) (2)16-22;/h3-13,16,18H,14-1,17H2,1-2H3;1H c1cc(c2c(c([nh]n2)ccc3)c3c4=o)c4cc1 O=C1c2ccccc2c3n[nH]c4cccc1c34 InChI=1/C14H8N2O/c (9) (14) C14H8N2O (12) /h1-7H,(H,1,16) c1(cc(nc(c2cccs2)cn3)c3c4)c4n(c)c(c)n1 Cc1nc2cc3nc(cnc3cc2n1C)c4cccs4 InChI=1/C1H12N4S/c (7-14(12)19(9)2)16-8- C1H12N4S (18-11) /h3-8H,1-2H3 n1c(n)c(c(c2cccc(br)c2)cc(c3ccc(n4ccocc4)nc3)n)cnc1.[h Cl.Cl.Nc1ncnc2nc(cc(c3cccc(Br)c3)c12)c4ccc(nc4)NCCOCC InChI=1/C22H19BrN6O.2ClH/c (1-16) C22H21BrCl2N6O ]Cl.[H]Cl 18( (17)21(24) ) (2-12-1) ;;/h1-,1-13H,6-9H2,(H2,24,26,27,28);2*1H c1(c2nc(oc)ncc2)n(c3ccc(o)cc3)cnc1c4ccc(f)cc4 COc1nccc(n1)c2c(ncn2C3CCC(O)CC3)c4ccc(F)cc4 InChI=1/C2H21FN4O2/c (24-2)19-18(13- C2H21FN4O (21)-3-13) (19) (26)9-7-1/h2-,1-12,1-16,26H,6-9H2,1H3 c1ccc(cn(c\c=c\c2ccc(cl)cc2)c)c((=o)(=o)c3ccc(oc)cc3)c COc1ccc(cc1)S(=O)(=O)Nc2ccccc2CN(C)C\C=C\c3ccc(Cl)cc3. InChI=1/C24H2ClN2O3S.H3O4P/c1-27( C24H28ClN2O7PS OP(=O)(O)O OP(=O)(O)O 21(2) ) (2)26-31(28,29) (3-2) ;1-(2,3)4/h3-16,26H,17-18H2,1-2H3;(H3,1,2,3,4)/b6-+; n1c(ncc2ccc([n+]([o- [O- InChI=1/C2H16F3N7O2/c21-2(22,23) (8-14)1- C2H16F3N7O ])=O)cc2)c(nc[nH]3)c3nc1NCc4cc(C(F)(F)F)ccc4 ][N+](=O)c1ccc(CNc2nc(NCc3cccc(c3)C(F)(F)F)nc4[nH]cnc24)c (16-18(29-19) ) (7-- c1 12)3(31)32/h1-8,11H,9-1H2,(H3,24,2,26,27,28,29) c1(br)cc(\c=c(/c#n)\c#n)cc(c(c)(c)c)c1o CC(C)(C)c1cc(C=C(C#N)C#N)cc(Br)c1O InChI=1/C14H13BrN2O/c1-14(2,3)11--9(4-1(7-16)8-17)6- C14H13BrN2O (1)13(11)18/h4-6,18H,1-3H3 n1c(n)c(cc2ccc(occ3ccc(oc)cc3)c(oc)c2)cnc1n COc1ccc(COc2ccc(Cc3cnc(N)nc3N)cc2OC)cc1 InChI=1/C2H22N4O3/c (4-7-16) C2H22N4O (1-18(17)26-2) (22)24-19(1)21/h3-8,1-11H,9,12H2,1-2H3,(H4,21,22,23,24) c1(nc(c#cc)=o)cc(c(nc2ccc(f)c(cl)c2)ncn3)c3cn1 CC#CC(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cn1 InChI=1/C17H11ClFNO/c (2) (8-2- C17H11ClFNO ) (11) (19)12(18)6-1/h4-9H,1H3,(H,2,24,2)(H,21,22,23) n1c(nc2cccc(cl)c2)c(ncn3cc)c3nc1n[c@h]4[c@@h](n)ccc CCn1cnc2c(Nc3cccc(Cl)c3)nc(N[C@@H]4CCCC[C@@H]4N)n InChI=1/C19H24ClN7/c ( C19H24ClN C4 c12 12(2)1-13)2-19(26-18(16)27) (1)21/h- 7,1-11,14-1H,2-4,8-9,21H2,1H3,(H2,23,24,2,26)/t14-,1+/m/s1 C1Cc(c(C#N)c(NC(=O)c2c(cccc3)c3ccc2)s4)c4CC1.O([H])[H] O.O=C(Nc1sc2CCCCc2c1C#N)c3cccc4ccccc34 InChI=1/C2H16N2OS.H2O/c (1)24- C2H18N2O2S (17)22-19(23) (13)16;/h1-2,- 8,1H,3-4,9,11H2,(H,22,23);1H2 C1(N(C)C(=O)C(c(c2c([nH]3)cccc2)c3)=C1c(c4c([nH])cccc4)c CN1C(=O)C(=C(C1=O)c2c[nH]c3ccccc23)c4c[nH]cccccc4 InChI=1/C21H1N3O2/c1-24-2(2)18( C21H1N3O )=O 12(14)16)19(21(24)26) (1)17/h2-11,22-23H,1H3 c1(nc(=o)cc)cc(c(nc2cccc(br)c2)ncn3)c3cc1 CCC(=O)Nc1ccc2ncnc(Nc3cccc(Br)c3)c2c1 InChI=1/C17H1BrN4O/c1-2-16(23) (9- C17H1BrN4O )17( ) (18)8-12/h3-1H,2H2,1H3,(H,21,23)(H,19,2,22) c1c(c(=o)c=c(n2ccncc2)o3)c3c(c4ccccc4)cc1 O=C1C=C(Oc2c1cccc2c3ccccc3)N4CCNCC4 InChI=1/C19H18N2O2/c ( )23- C19H18N2O ( (17)19) /h1-8,13,2H,9-12H2 c1c(c(ccc(cc(oco2)c2c3)c34)c4[n+](c)c)cc(oc)c(oc)c1.[cl- [Cl-].COc1ccc2c(c[n+](C)c3c2ccc4ccOCOccc34)c1OC InChI=1/C21H18NO4.ClH/c (6-7-17(23- C21H18ClNO ] 2)21(16)24-3) ( )9-1(12)2(14)22;/h4-1H,11H2,1-3H3;1H/q+1;/p-1
19 DMBI p21-activated Kinase Inhibitor III, IPA DNA-PK Inhibitor II PI Casein Kinase I Inhibitor, D PDGF Receptor Tyrosine Kinase Inhibitor II p38 MAP Kinase Inhibitor III VEGF Receptor 2 Kinase Inhibitor II PD Necrostatin Scytonemin, Lyngbya sp PKCbII/EGFR Inhibitor Polo-like Kinase Inhibitor I VEGF Receptor 2 Kinase Inhibitor IV AG IGF-1R Inhibitor II HA 14, Dihydrochloride Cyano-3-methylisoquinoline p38 MAP Kinase Inhibitor IV Diacylglycerol Kinase Inhibitor II Cdk9 Inhibitor II Syk Inhibitor III Cdk4 Inhibitor II, PIM1/2 Kinase Inhibitor V ERK Inhibitor II, Negative Control LY PI 3-Kγ Inhibitor VII SB Cdk2 Inhibitor II PP1 Analog II, 1NM-PP Wee1 Inhibitor II IKK-2 Inhibitor VIII Stem-Cell Factor/c-Kit Inhibitor, ISCK TGF-b RI Inhibitor III KN p38 MAP Kinase Inhibitor VI, JX CaMKII Inhibitor, CK Cdk1 Inhibitor PI 3-K&gamme;/CKII Inhibitor Syk Inhibitor II VEGF Receptor Tyrosine Kinase Inhibitor II AMPK Inhibitor, Compound C c1(\c=c(\c2=o)/c(cccc3)c3n2)ccc(n(c)c)cc1 CN(C)c1ccc(\C=C/2\C(=O)Nc3ccccc23)cc1 InChI=1/C17H16N2O/c1-19(2) (8-1-13) C17H16N2O (14)18-17(1)2/h3-11H,1-2H3,(H,18,2)/b1-11+ c1cc(cccc2)c2c(ssc3c(o)ccc(cccc4)c34)c1o Oc1ccc2ccccc2c1SSc3c(O)ccc4ccccc34 InChI=1/C2H14O2S2/c (13)19(17)23- C2H14O2S (16) (2)22/h1-12,21-22H c1(oc(n2ccocc2)=cc3=o)c3ccc(cccc4)c14 O=C1C=C(Oc2c1ccc3ccccc23)N4CCOCC4 InChI=1/C17H1NO3/c ( ) C17H1NO (13)-6-14(1)17/h1-6,11H,7-1H2 c1(c(n2ccocc2)nc(c3cc(o)ccc3)n4)c4c(cccn)co1 Oc1cccc(c1)c2nc(N3CCOCC3)c4ocnccccc4n2 InChI=1/C19H16N4O3/c (11-13) C19H16N4O (14)26-16(1)18(22-17) /h1-6,11,24H,7-1H2 c1(c2ccc(occo3)c3c2)nc(c4ccc(c(n)=o)cc4)[nh]c1cncccc NC(=O)c1ccc(cc1)c2nc(c3ccc4OCCOc4c3)c([nH]2)cccccn InChI=1/C23H18N4O3/c24-22(28) (7--14) C23H18N4O (21(27-23) ) (13-16) /h1-1,13H,11-12H2,(H2,24,28)(H,26,27) c1(oc(=o)ccc)cc(cc(c(=o)c2[nh]c3c(c2)cccc3)[nh]4)c4cc1 CCCC(=O)Oc1ccc2[nH]c(cc2c1)C(=O)c3cc4ccccc4[nH]3 InChI=1/C21H18N2O3/c1-2--2(24) (1-1)12- C21H18N2O (23-17)21(2) (13)22-18/h3-4,6-12,22-23H,2,H2,1H3 c1(c2cc(nc(c3ccccc3)c)ncc2)[nh]c(sc)nc1c4ccc(f)cc4 CSc1nc(c2ccc(F)cc2)c([nH]1)c3ccnc(NC(C)c4ccccc4)c3 InChI=1/C23H21FN4S/c1-1( ) (12- C23H21FN4S )22-21(27-23(28-22)29-2) (24) /h3-1H,1-2H3,(H,2,26)(H,27,28) c1(br)cc(\c(=c\c2cc(cccc3)c3[nh]2)\c(=o)n4)c4cc1 Brc1ccc2NC(=O)\C(=C/c3cc4CCCCc4[nH]3)\c2c1 InChI=1/C17H1BrN2O/c (8-11)14(17(21)2- C17H1BrN2O ) (1)19-12/h-9,19H,1-4H2,(H,2,21)/b14-9- c1(c2ccc([n+]([o-])=o)cc2)[nh]c(c(ccnc3)c3)c(c4ccc(f)cc4)n1 [O-][N+](=O)c1ccc(cc1)c2nc(c3ccc(F)cc3)c([nH]2)c4ccncc4 InChI=1/C2H13FN4O2/c (2-6-16)18-19( C2H13FN4O )24-2(23-18) (8-4-1)2(26)27/h1-12H,(H,23,24) c1cc(c(cc2c(=o)n(c)c(=s)n2)c[nh]3)c3cc1 CN1C(=S)NC(Cc2c[nH]c3ccccc23)C1=O InChI=1/C13H13N3OS/c (17)11(1-13(16)18) C13H13N3OS (8)1/h2-,7,11,14H,6H2,1H3,(H,1,18) c1cccc(c(=c2c3=c4c(=nc(cccc)c4)\c(=c([h])/c6ccc(o)cc6)\ Oc1ccc(\C=C/2\C(=O)C(=C3C2=Nc4ccccc34)C=C6C(=Nc7ccc InChI=1/C36H2N2O4/c ( ) C36H2N2O C3=O)C(\C(=C([H])/c7ccc(O)cc7)\C2=O)=N8)c18 cc67)\c(=c/c8ccc(o)cc8)\c=o)cc1 29( (23)37-33)31(3(2)41) (24)38-34(3)26(36(32)42) (4) /h1-18,39-4H/b2-17+, C1(NC(=O)c2c1cc(Nc3ccc(F)cc3)c(Nc4ccc(F)cc4)c2)=O Fc1ccc(Nc2cc3C(=O)NC(=O)c3cc2Nc4ccc(F)cc4)cc1 InChI=1/C2H13F2N3O2/c (6-2-11) C2H13F2N3O (2(27)2-19(1)26)1-18(17) (22)4-8-14/h1-1,23-24H,(H,2,26,27) c1c(occ2ccc(s(c)(=o)=o)cc2)c(c(=o)n)sc1n3cnc(cc(oc)c(o COc1cc2ncn(c3cc(OCc4ccc(cc4)S(=O)(=O)C)c(s3)C(=O)N)c2c InChI=1/C22H21N3O6S2/c (9-18(17)3- C22H21N3O6S C)c4)c34 c1oc 2)2( )2-1-19(21(32-2)22(23)26) (7- -13)33(3,27)28/h4-1,12H,11H2,1-3H3,(H2,23,26) c1(c2ccc(oc)cc2)cn(ncc3c4ccsc4)c3nc1 COc1ccc(cc1)c2cnc3c(cnn3c2)c4ccsc4 InChI=1/C17H13N3OS/c (3--1) C17H13N3OS (9-19-2(17)1-14) /h2-11H,1H3 n1c(c2nc(c3ccccc3)c1)cc(oc)c(oc)c2 COc1cc2ncc(nc2cc1OC)c3ccccc3 InChI=1/C16H14N2O2/c (9-16(1)2-2)18- C16H14N2O ( ) /h3-1H,1-2H3 c1c(cl)cc(nc(nc2c3c(cccc3)nc(c)c2)=o)c(oc)c1 COc1ccc(Cl)cc1NC(=O)Nc2cc(C)nc3ccccc23 InChI=1/C18H16ClN3O2/c ( (13)2- C18H16ClN3O )21-18(23) (19)7-8-17(16)24-2/h3-1H,1-2H3,(H2,2,21,22,23) c1cc(c(s(=o)(=o)nccnc(n)=n)ccc2)c2cn1.[h]cl.[h]cl Cl.Cl.NC(=N)NCC(=O)(=O)c1cccc2cnccc12 InChI=1/C12H1NO2S.2ClH/c13-12(14) C12H17Cl2NO2S (18,19) (9)11;;/h1-,8,17H,6-7H2,(H4,13,14,16);2*1H c1cc(c(c#n)c(c)nc2)c2cc1 Cc1ncc2ccccc2c1C#N InChI=1/C11H8N2/c1-8-11(6-12) (1)7-13-8/h2- C11H8N ,7H,1H3 c1c(cl)c(cl)c(s(=o)(=o)c2c(cl)c(cl)cc(cl)c2o)c(o)c1cl Oc1c(Cl)cc(Cl)c(Cl)c1S(=O)(=O)c2c(O)c(Cl)cc(Cl)c2Cl InChI=1/C12H4Cl6O4S/c C12H4Cl6O4S (1)9(19)11(7(3)17)23(21,22)12-8(18)4(14)2-6(16)1(12)2/h1-2,19-2H N1c(c2C(=O)N(CCN3CC\C(=C(/c4ccc(F)cc4)\cccc(F)cc)\CC3 Fc1ccc(cc1)C(=C2CCN(CCN3C(=S)Nc4ccccc4C3=O)CC2)ccc InChI=1/C28H2F2N3OS/c (6-1-22)26( C28H2F2N3OS )C1=S)cccc2 c(f)cc 23(3)12-8-2) ( ) (34) (24)31-28(33)3/h1-12H,13-18H2,(H,31,3) c1(n=nc2ccc(o)cc2)c(n)n[nh]c1n Nc1n[nH]c(N)c1N=Nc2ccc(O)cc2 InChI=1/C9H1N6O/c1-8-7(9(11)1-14-8) (16)4- C9H1N6O /h1-4,16H,(H,1,11,14,1)/b c1(cc(\c=c\[n+]([o-])=o)cc2)c2oco1 [O-][N+](=O)\C=C\c1ccc2OCOc2c1 InChI=1/C9H7NO4/c11-1(12) (-7) /h1- C9H7NO H,6H2/b4-3+ c1c(c(=s)c(c(oc)ccc2oc)c2n3)c3ccc1 COc1ccc(OC)c2C(=S)c3ccccc3Nc12 InChI=1/C1H13NO2S/c (18-2)14-13(11)1(19)9- C1H13NO2S (9)16-14/h3-8H,1-2H3,(H,16,19) c1(c(f)(f)f)cc(\c=c(\c(=o)nc2=o)/s2)ccc1 FC(F)(F)c1cccc(\C=C\2/SC(=O)NC2=O)c1 InChI=1/C11H6F3NO2S/c12-11(13,14) (4-7)-8- C11H6F3NO2S (16)1-1(17)18-8/h1-H,(H,1,16,17)/b8-- c1cc(c(c2nnc([nh]nc3o)c3c2)c(c4ccccc4)n)ncc1 Oc1n[nH]c2nnc(cc12)c3c(nn4ccccc34)cccccc InChI=1/C18H12N6O/c ( (12) C18H12N6O ) (14)23-16(1) /h1-1H,(H2,2,21,22,2) C(N1CCOCC1)(Oc(c2c3ccccc3)c(ccc2)C4=O)=C4 O=C1C=C(Oc2c1cccc2c3ccccc3)N4CCOCC4 InChI=1/C19H17NO3/c ( )23- C19H17NO ( (17)19) /h1-8,13H,9-12H2 c1(cc(\c=c(\c(=o)nc2=o)/s2)cc3)c3oco1 O=C1NC(=O)\C(=C\c2ccc3OCOc3c2)\S1 InChI=1/C11H7NO4S/c13-1-9(17-11(14)12-1) (3- C11H7NO4S ) /h1-4H,H2,(H,12,13,14)/b9-4- c1(c2ccc(s(c)=o)cc2)[nh]c(c(ccnc3)c3)c(c4ccc(f)cc4)n1 CS(=O)c1ccc(cc1)c2nc(c3ccc(F)cc3)c([nH]2)c4ccncc4 InChI=1/C21H16FN3OS/c1-27(26) (-9-18) C21H16FN3OS ( (22)7-3-14)2(2-21) /h2-13H,1H3,(H,24,2) c1(br)cc(\c(=n\nc2ccc(s(n)(=o)=o)cc2)\c(=o)n3)c3cc1 (=O)(=O)c1ccc(N\N=C\2/C(=O)Nc3ccc(Br)cc23)cc1 InChI=1/C14H11BrN4O3S/c (7-8)13(14(2)17- C14H11BrN4O3S ) (-3-9)23(16,21)22/h1-7,18H,(H2,16,21,22)(H,17,19,2) c1(n)c(c(cc2cccc(cccc3)c23)nn4c(c)(c)c)c4ncn1 CC(C)(C)n1nc(Cc2cccc3ccccc23)c4c(N)ncnc14 InChI=1/C2H21N/c1-2(2,3) (18(21) C2H21N )16(24-2) (13)14/h4-1,12H,11H2,1-3H3,(H2,21,22,23) c1(c(c(=o)nc2=o)c2c(c3ccccc3cl)c4)c4n(cccc)c(c1)ccc(o) CCCCn1c2ccc(O)cc2c3c4C(=O)NC(=O)c4c(cc13)ccccccCl InChI=1/C24H19ClN2O3/c (28)11- C24H19ClN2O c 16(18)2-19(27)12-1( (14)2)21-22(2)24(3)26-23(21)29/h4-9,11-12,28H,2-3,1H2,1H3,(H,26,29,3) c1c(c2ccncc2)c(c#n)c(n)nc1c3c(o)cccc3occ4cc4 Nc1nc(cc(C2CCNCC2)c1C#N)c3c(O)cccc3OCC4CC4 InChI=1/C21H24N4O2/c ( )1- C21H24N4O (2-21(16)23)2-18(26) (2) /h1-3,1,13-14,24,26H,4-9,12H2,(H2,23,2) n1cn(c2ccc((=o)(=o)c3ccc(c(c)(c)c)cc3)cc2)cc1 CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc2ccc(cc2)n3ccnc3 InChI=1/C19H21N3O2S/c1-19(2,3) (11-- C19H21N3O2S )2(23,24) (9-7-16) /h4-14,21H,1-3H3 c1(c2ccc(oco3)c3c2)[nh]c(c(c)(c)c)nc1c4nc(c)ccc4.cl Cl.Cc1cccc(n1)c2nc([nH]c2c3ccc4OCOc4c3)C(C)(C)C InChI=1/C2H21N3O2.ClH/c (21-12)18-17(22- C2H22ClN3O (23-18)2(2,3)4) (1-13) ;/h- 1H,11H2,1-4H3,(H,22,23);1H n1cc(cccc2osooc3ccc(cc(n(s(=o)(=o)c4cccc(cncc)c4)c) CN(C(Cc1ccc(OOSOc2cccc3cnccc23)cc1)C(=O)N4CCN(CC4)c InChI=1/C38H3NO6S2/c1-41(1(4,46) C38H3NO6S C(=O)N6CCN(c7ccccc7)CC6)cc3)c2cc1 ccccc)s(=o)(=o)c6cccc7cnccc (3)37)3(38(44) ( ) ) ( ) (29)36/h2-2,26-27,3H,21-2H2,1H3 c1(oc)c(c(n2ccc(cc3ccccc3)cc2)=o)ccc(sc)c1 COc1cc(SC)ccc1C(=O)N2CCC(Cc3ccccc3)CC2 InChI=1/C21H2NO2S/c (2-2)8-9- C21H2NO2S (2)21(23) ( ) /h3-9,1,17H,1-14H2,1-2H3 n1c(nccccccnc(=o)oc(c)(c)c)c(ncn2c(c)c)c2nc1ncco CC(C)n1cnc2c(NCCCCCCNC(=O)OC(C)(C)C)nc(NCCO)nc12 InChI=1/C21H37N7O3/c1-1(2) (26-19( C21H37N7O )27-18(16)28) (3)31-21(3,4)/h14-1,29H,6-13H2,1-H3,(H,24,3)(H2,22,23,26,27) c1cc(\c(=c/c2c(cl)[nh]c(c3)c2ccc3)\c(=o)n4)c4cc1 Clc1[nH]c2ccccc2c1\C=C/3\C(=O)Nc4ccccc34 InChI=1/C17H11ClN2O/c ( (1)19-16)9- C17H11ClN2O (11)2-17(13)21/h1-9,19H,(H,2,21)/b13-9+ c1cc(c2c(o)cc(f)cc2)oc1\c=c(/sc(=o)n3)\c3=o Oc1cc(F)ccc1c2oc(\C=C\3/SC(=O)NC3=O)cc2 InChI=1/C14H8FNO4S/c (1(17)-7) (2- C14H8FNO4S ) (18)16-14(19)21-12/h1-6,17H,(H,16,18,19)/b12-6- n1c(nc2cccc(c(f)(f)f)c2)c(c(n)=o)cnc1nccn.cl.cl.o.o O.O.Cl.Cl.NCCNc1ncc(C(=O)N)c(Nc2cccc(c2)C(F)(F)F)n1 InChI=1/C14H1F3N6O.2ClH.2H2O/c1-14(16,17) (6- C14H21Cl2F3N6O ) (11(19)24) (23-12) ;;;;/h1-3,6-7H,4-,18H2,(H2,19,24)(H2,2,21,22,23);2*1H;2*1H2 c1cc(c(=o)nc2ccc(cl)cc2)c(ncc3ccncc3)cc1 Clc1ccc(NC(=O)c2ccccc2NCc3ccncc3)cc1 InChI=1/C19H16ClN3O/c (8-6-1)23-19(24) C19H16ClN3O (17) /h1-12,22H,13H2,(H,23,24) c1(c2ccc(occn3ccccc3)cc2)cn(ncc4cccncc)c4nc1 C(CN1CCCCC1)Oc2ccc(cc2)c3cnc4c(cnn4c3)cccncc InChI=1/C24H2NO/c (13-3-1) (- C24H2NO ) ( (24)18-21) /h4-11,16-18H,1-3,12-1H2
encouraged to use the Version of Record that, when published, will replace this version. The most /BCJ BIOCHEMICAL JOURNAL
 Biochemical Journal: this is an Accepted Manuscript, not the final Version of Record. You are encouraged to use the Version of Record that, when published, will replace this version. The most up-to-date
Biochemical Journal: this is an Accepted Manuscript, not the final Version of Record. You are encouraged to use the Version of Record that, when published, will replace this version. The most up-to-date
Supplementary Table 1. Primers used for RT-qPCR analysis of striatal and nigral tissue.
 Supplementary Table 1. Primers used for RT-qPCR analysis of striatal and nigral tissue. Gene Forward Primer (5-3 ) Reverse Primer (5-3 ) Dopaminergic Markers TH CTG GCC ATT GAT GTA CTG GA ACA CAC ATG GGA
Supplementary Table 1. Primers used for RT-qPCR analysis of striatal and nigral tissue. Gene Forward Primer (5-3 ) Reverse Primer (5-3 ) Dopaminergic Markers TH CTG GCC ATT GAT GTA CTG GA ACA CAC ATG GGA
encouraged to use the Version of Record that, when published, will replace this version. The most /BCJ
 Biochemical Journal: this is an Accepted Manuscript, not the final Version of Record. You are encouraged to use the Version of Record that, when published, will replace this version. The most up-to-date
Biochemical Journal: this is an Accepted Manuscript, not the final Version of Record. You are encouraged to use the Version of Record that, when published, will replace this version. The most up-to-date
Supporting Information
 Chloromethylhalicyclamine B, a Marine-Derived Protein Kinase CK1δ/ε Inhibitor Germana Esposito, Marie-Lise Bourguet-Kondracki, * Linh H. Mai, Arlette Longeon, Roberta Teta, Laurent Meijer, Rob Van Soest,
Chloromethylhalicyclamine B, a Marine-Derived Protein Kinase CK1δ/ε Inhibitor Germana Esposito, Marie-Lise Bourguet-Kondracki, * Linh H. Mai, Arlette Longeon, Roberta Teta, Laurent Meijer, Rob Van Soest,
< (0.999) Graft (0.698) (0.483) <0.001 (0.698) (<0.001) (<0.001) 3 months (0.999) (0.483) (<0.001) 6 months (<0.
 Supplementary table 1. Correlation of endothelial cell density among graft, 3, 6, and 12 months after Descemet s automated stripping endothelial keratoplasty. Graft 3 months 6 months 12 months Graft
Supplementary table 1. Correlation of endothelial cell density among graft, 3, 6, and 12 months after Descemet s automated stripping endothelial keratoplasty. Graft 3 months 6 months 12 months Graft
A strategy for the identification of combinatorial bioactive compounds. contributing to the holistic effect of herbal medicines
 1 2 Supplementary information 3 4 A strategy for the identification of combinatorial bioactive compounds contributing to the holistic effect of herbal medicines 5 6 Fang Long 1, Hua Yang 1, Yanmin Xu,
1 2 Supplementary information 3 4 A strategy for the identification of combinatorial bioactive compounds contributing to the holistic effect of herbal medicines 5 6 Fang Long 1, Hua Yang 1, Yanmin Xu,
Μελέτη της έκφρασης του ογκοκατασταλτικού γονιδίου Cyld στον καρκίνο του μαστού
 Σχολή Θετικών Επιστημών Τμήμα Βιολογίας Πρόγραμμα Μεταπτυχιακών Σπουδών Κατεύθυνση: Εφαρμοσμένη γενετική και βιοτεχνολογία ΜΕΤΑΠΤΥΧΙΑΚΗ ΔΙΠΛΩΜΑΤΙΚΗ ΕΡΓΑΣΙΑ Μελέτη της έκφρασης του ογκοκατασταλτικού γονιδίου
Σχολή Θετικών Επιστημών Τμήμα Βιολογίας Πρόγραμμα Μεταπτυχιακών Σπουδών Κατεύθυνση: Εφαρμοσμένη γενετική και βιοτεχνολογία ΜΕΤΑΠΤΥΧΙΑΚΗ ΔΙΠΛΩΜΑΤΙΚΗ ΕΡΓΑΣΙΑ Μελέτη της έκφρασης του ογκοκατασταλτικού γονιδίου
Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes
![Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes](/thumbs/92/108867714.jpg) SUPPORTING INFORMATION Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Eric J. Uzelac, Casey B. McCausland, and Seth C. Rasmussen*
SUPPORTING INFORMATION Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Eric J. Uzelac, Casey B. McCausland, and Seth C. Rasmussen*
BD Cytometric Bead Array Flex Set System
 BD Cytometric Bead Array Flex Set System BD Cytometric Bead Array System BD Biosciences Pharmingen ELISA Cytometric Bead Array CBA System Capture Caputure Serum Supernatant Lysate PE -Detection 1 2 PE
BD Cytometric Bead Array Flex Set System BD Cytometric Bead Array System BD Biosciences Pharmingen ELISA Cytometric Bead Array CBA System Capture Caputure Serum Supernatant Lysate PE -Detection 1 2 PE
Supplementary Information Titles
 Supplementary Information Titles Journal: Nature Neuroscience Article Title: Corresponding Author: Wnt-mediated activation of NeuroD1 and retroelements during Tomoko Kuwabara adult neurogenesis Supplementary
Supplementary Information Titles Journal: Nature Neuroscience Article Title: Corresponding Author: Wnt-mediated activation of NeuroD1 and retroelements during Tomoko Kuwabara adult neurogenesis Supplementary
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Cyclopentadienyl iron dicarbonyl (CpFe(CO) 2 ) derivatives
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Cyclopentadienyl iron dicarbonyl (CpFe(CO) 2 ) derivatives
ΜΟΡΙΑΚΕΣ ΜΕΘΟΔΟΙ ΚΡΙΤΗΡΙΑ ΕΠΙΛΟΓΗΣ ΑΞΙΟΛΟΓΗΣΗ
 - - ό ό : Ω ί ό ί ώ.. ά ή ί ός ή ς ύ ί ί ή έ ώ ό. ή ς ά ς ής ό ός ά ς ή έ ός ή ς ί ής ί ς. ό έ ώ - ώ ή ής ή ς- ί ά ά ς ί ς. ά ί ί έ έ ά ύ ή, ό ί ά, ό ό ά έ ά ής ί ύ George Wald ή ί έ ς ί ύ ό ς ί ς ά έ
- - ό ό : Ω ί ό ί ώ.. ά ή ί ός ή ς ύ ί ί ή έ ώ ό. ή ς ά ς ής ό ός ά ς ή έ ός ή ς ί ής ί ς. ό έ ώ - ώ ή ής ή ς- ί ά ά ς ί ς. ά ί ί έ έ ά ύ ή, ό ί ά, ό ό ά έ ά ής ί ύ George Wald ή ί έ ς ί ύ ό ς ί ς ά έ
ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΤΜΗΜΑ ΟΔΟΝΤΙΑΤΡΙΚΗΣ ΕΡΓΑΣΤΗΡΙΟ ΟΔΟΝΤΙΚΗΣ ΚΑΙ ΑΝΩΤΕΡΑΣ ΠΡΟΣΘΕΤΙΚΗΣ
 ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΤΜΗΜΑ ΟΔΟΝΤΙΑΤΡΙΚΗΣ ΕΡΓΑΣΤΗΡΙΟ ΟΔΟΝΤΙΚΗΣ ΚΑΙ ΑΝΩΤΕΡΑΣ ΠΡΟΣΘΕΤΙΚΗΣ ΣΥΓΚΡΙΤΙΚΗ ΜΕΛΕΤΗ ΤΗΣ ΣΥΓΚΡΑΤΗΤΙΚΗΣ ΙΚΑΝΟΤΗΤΑΣ ΟΡΙΣΜΕΝΩΝ ΠΡΟΚΑΤΑΣΚΕΥΑΣΜΕΝΩΝ ΣΥΝΔΕΣΜΩΝ ΑΚΡΙΒΕΙΑΣ
ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΤΜΗΜΑ ΟΔΟΝΤΙΑΤΡΙΚΗΣ ΕΡΓΑΣΤΗΡΙΟ ΟΔΟΝΤΙΚΗΣ ΚΑΙ ΑΝΩΤΕΡΑΣ ΠΡΟΣΘΕΤΙΚΗΣ ΣΥΓΚΡΙΤΙΚΗ ΜΕΛΕΤΗ ΤΗΣ ΣΥΓΚΡΑΤΗΤΙΚΗΣ ΙΚΑΝΟΤΗΤΑΣ ΟΡΙΣΜΕΝΩΝ ΠΡΟΚΑΤΑΣΚΕΥΑΣΜΕΝΩΝ ΣΥΝΔΕΣΜΩΝ ΑΚΡΙΒΕΙΑΣ
PHOS π 0 analysis, for production, R AA, and Flow analysis, LHC11h
 PHOS π, ask PHOS π analysis, for production, R AA, and Flow analysis, Henrik Qvigstad henrik.qvigstad@fys.uio.no University of Oslo --5 PHOS π, ask ask he task we use, AliaskPiFlow was written prior, for
PHOS π, ask PHOS π analysis, for production, R AA, and Flow analysis, Henrik Qvigstad henrik.qvigstad@fys.uio.no University of Oslo --5 PHOS π, ask ask he task we use, AliaskPiFlow was written prior, for
Christopher Stephen Inchley, 2015
 Christopher Stephen Inchley, 2015 Series of dissertations submitted to the Faculty of Medicine, University of Oslo No. 2009 ISBN 978-82-8264-990-2 All rights reserved. No part of this publication may be
Christopher Stephen Inchley, 2015 Series of dissertations submitted to the Faculty of Medicine, University of Oslo No. 2009 ISBN 978-82-8264-990-2 All rights reserved. No part of this publication may be
α α Pneumocystis jirovecii
 α α Key words α α Pneumocystis jirovecii I α Table 1. Biologics currently approved in Japan for autoimmune inflammatory diseases (as of Dec, 2016) Classification Preparations that target cytokines or cytokine
α α Key words α α Pneumocystis jirovecii I α Table 1. Biologics currently approved in Japan for autoimmune inflammatory diseases (as of Dec, 2016) Classification Preparations that target cytokines or cytokine
ΠΡΟΣΚΛΗΣΗ ΕΚΔΗΛΩΣΗΣ ΕΝΔΙΑΦΕΡΟΝΤΟΣ
 1 ΕΛΛΗΝΙΚΗ ΔΗΜΟΚΡΑΤΙΑ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΡΗΤΗΣ ΕΙΔΙΚΟΣ ΛΟΓΑΡΙΑΣΜΟΣ ΑΝΑΡΤΗΤΕΑ ΣΤΟ ΔΙΑΔΙΚΤΥΟ ΠΡΟΣΚΛΗΣΗ ΕΚΔΗΛΩΣΗΣ ΕΝΔΙΑΦΕΡΟΝΤΟΣ Ηράκλειο, 04.02.2014 Αρ. πρωτ. 1054 Ο Ειδικός Λογαριασμός του Πανεπιστημίου Κρήτης
1 ΕΛΛΗΝΙΚΗ ΔΗΜΟΚΡΑΤΙΑ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΡΗΤΗΣ ΕΙΔΙΚΟΣ ΛΟΓΑΡΙΑΣΜΟΣ ΑΝΑΡΤΗΤΕΑ ΣΤΟ ΔΙΑΔΙΚΤΥΟ ΠΡΟΣΚΛΗΣΗ ΕΚΔΗΛΩΣΗΣ ΕΝΔΙΑΦΕΡΟΝΤΟΣ Ηράκλειο, 04.02.2014 Αρ. πρωτ. 1054 Ο Ειδικός Λογαριασμός του Πανεπιστημίου Κρήτης
Cellular Physiology and Biochemistry
 Original Paper 2016 The Author(s). 2016 Published The Author(s) by S. Karger AG, Basel Published online: November 25, 2016 www.karger.com/cpb Published by S. Karger AG, Basel 486 www.karger.com/cpb Accepted:
Original Paper 2016 The Author(s). 2016 Published The Author(s) by S. Karger AG, Basel Published online: November 25, 2016 www.karger.com/cpb Published by S. Karger AG, Basel 486 www.karger.com/cpb Accepted:
Supporting information. An unusual bifunctional Tb-MOF for highly sensing of Ba 2+ ions and remarkable selectivities of CO 2 /N 2 and CO 2 /CH 4
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information An unusual bifunctional Tb-MOF for highly sensing
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information An unusual bifunctional Tb-MOF for highly sensing
ΓΕΩΠΟΝΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΑΘΗΝΩΝ ΤΜΗΜΑ ΑΓΡΟΤΙΚΗΣ ΟΙΚΟΝΟΜΙΑΣ & ΑΝΑΠΤΥΞΗΣ
 ΓΕΩΠΟΝΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΑΘΗΝΩΝ ΤΜΗΜΑ ΑΓΡΟΤΙΚΗΣ ΟΙΚΟΝΟΜΙΑΣ & ΑΝΑΠΤΥΞΗΣ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ «ΟΛΟΚΛΗΡΩΜΕΝΗ ΑΝΑΠΤΥΞΗ & ΔΙΑΧΕΙΡΙΣΗ ΤΟΥ ΑΓΡΟΤΙΚΟΥ ΧΩΡΟΥ» ΜΕΤΑΠΤΥΧΙΑΚΗ ΕΡΓΑΣΙΑ «Οικονομετρική διερεύνηση
ΓΕΩΠΟΝΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΑΘΗΝΩΝ ΤΜΗΜΑ ΑΓΡΟΤΙΚΗΣ ΟΙΚΟΝΟΜΙΑΣ & ΑΝΑΠΤΥΞΗΣ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ «ΟΛΟΚΛΗΡΩΜΕΝΗ ΑΝΑΠΤΥΞΗ & ΔΙΑΧΕΙΡΙΣΗ ΤΟΥ ΑΓΡΟΤΙΚΟΥ ΧΩΡΟΥ» ΜΕΤΑΠΤΥΧΙΑΚΗ ΕΡΓΑΣΙΑ «Οικονομετρική διερεύνηση
Τα ηπατικά επίπεδα του FOXP3 mrna στη χρόνια ηπατίτιδα Β εξαρτώνται από την έκφραση των οδών Fas/FasL και PD-1/PD-L1
 Τα ηπατικά επίπεδα του FOXP3 mrna στη χρόνια ηπατίτιδα Β εξαρτώνται από την έκφραση των οδών Fas/FasL και PD-1/PD-L1 Γ. Γερμανίδης, Ν. Αργέντου, Θ. Βασιλειάδης, Κ. Πατσιαούρα, Κ. Μαντζούκης, A. Θεοχαρίδου,
Τα ηπατικά επίπεδα του FOXP3 mrna στη χρόνια ηπατίτιδα Β εξαρτώνται από την έκφραση των οδών Fas/FasL και PD-1/PD-L1 Γ. Γερμανίδης, Ν. Αργέντου, Θ. Βασιλειάδης, Κ. Πατσιαούρα, Κ. Μαντζούκης, A. Θεοχαρίδου,
encouraged to use the Version of Record that, when published, will replace this version. The most /BCJ
 Biochemical Journal: this is an Accepted Manuscript, not the final Version of Record. You are encouraged to use the Version of Record that, when published, will replace this version. The most up-to-date
Biochemical Journal: this is an Accepted Manuscript, not the final Version of Record. You are encouraged to use the Version of Record that, when published, will replace this version. The most up-to-date
Chapter 1. Fingolimod attenuates ceramide induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes
 Chapter 1 Fingolimod attenuates ceramide induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes 1 1 1 1 CHAPTER SUMMARY FINGOLIMOD ATTENUATES CERAMIDE PRODUCTION
Chapter 1 Fingolimod attenuates ceramide induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes 1 1 1 1 CHAPTER SUMMARY FINGOLIMOD ATTENUATES CERAMIDE PRODUCTION
Supporting Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Crotonols A and B, Two Rare Tigliane Diterpenoid
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Crotonols A and B, Two Rare Tigliane Diterpenoid
ΠΑΝΕΠΙΣΤΗΜΙΟ ΙΩΑΝΝΙΝΩΝ ΑΝΟΙΚΤΑ ΑΚΑΔΗΜΑΪΚΑ ΜΑΘΗΜΑΤΑ
 ΠΑΝΕΠΙΣΤΗΜΙΟ ΙΩΑΝΝΙΝΩΝ ΑΝΟΙΚΤΑ ΑΚΑΔΗΜΑΪΚΑ ΜΑΘΗΜΑΤΑ Παθοφυσιολογία ΙΙ Ογκολογία Υπεύθυνος μαθήματος: Καθηγητής Αλέξανδρος Α. Δρόσος Άδειες Χρήσης Το παρόν εκπαιδευτικό υλικό υπόκειται σε άδειες χρήσης Creative
ΠΑΝΕΠΙΣΤΗΜΙΟ ΙΩΑΝΝΙΝΩΝ ΑΝΟΙΚΤΑ ΑΚΑΔΗΜΑΪΚΑ ΜΑΘΗΜΑΤΑ Παθοφυσιολογία ΙΙ Ογκολογία Υπεύθυνος μαθήματος: Καθηγητής Αλέξανδρος Α. Δρόσος Άδειες Χρήσης Το παρόν εκπαιδευτικό υλικό υπόκειται σε άδειες χρήσης Creative
Group 2 Methotrexate 7.5 mg/week, increased to 15 mg/week after 4 weeks. Methotrexate 7.5 mg/week, increased to 15 mg/week after 4 weeks
 Group 1 Methotrexate 7.5 mg/week, increased to 15 mg/week after 4 weeks Group 2 Methotrexate 7.5 mg/week, increased to 15 mg/week after 4 weeks Sulfasalazine 2000-3000 mg/day Leflunomide 20 mg/day Infliximab
Group 1 Methotrexate 7.5 mg/week, increased to 15 mg/week after 4 weeks Group 2 Methotrexate 7.5 mg/week, increased to 15 mg/week after 4 weeks Sulfasalazine 2000-3000 mg/day Leflunomide 20 mg/day Infliximab
Second Order RLC Filters
 ECEN 60 Circuits/Electronics Spring 007-0-07 P. Mathys Second Order RLC Filters RLC Lowpass Filter A passive RLC lowpass filter (LPF) circuit is shown in the following schematic. R L C v O (t) Using phasor
ECEN 60 Circuits/Electronics Spring 007-0-07 P. Mathys Second Order RLC Filters RLC Lowpass Filter A passive RLC lowpass filter (LPF) circuit is shown in the following schematic. R L C v O (t) Using phasor
Οι απόψεις και τα συμπεράσματα που περιέχονται σε αυτό το έγγραφο, εκφράζουν τον συγγραφέα και δεν πρέπει να ερμηνευτεί ότι αντιπροσωπεύουν τις
 Οι απόψεις και τα συμπεράσματα που περιέχονται σε αυτό το έγγραφο, εκφράζουν τον συγγραφέα και δεν πρέπει να ερμηνευτεί ότι αντιπροσωπεύουν τις επίσημες θέσεις των εξεταστών. i ΠΡΟΛΟΓΟΣ ΕΥΧΑΡΙΣΤΙΕΣ Η παρούσα
Οι απόψεις και τα συμπεράσματα που περιέχονται σε αυτό το έγγραφο, εκφράζουν τον συγγραφέα και δεν πρέπει να ερμηνευτεί ότι αντιπροσωπεύουν τις επίσημες θέσεις των εξεταστών. i ΠΡΟΛΟΓΟΣ ΕΥΧΑΡΙΣΤΙΕΣ Η παρούσα
Supplementary Figure 1
 NSC N2d N5d TNFRSFa TNFRSFb RIPK RelA Madd I-TRAF/TANK GAPDH γ-actin Supplementary Figure A 2 hrs Day : Cell seeding Day 9: +ng/ml TNF-α Day 9: Fixation B Merge Nestin PH-3 Hoechst Acute TNF-α Control
NSC N2d N5d TNFRSFa TNFRSFb RIPK RelA Madd I-TRAF/TANK GAPDH γ-actin Supplementary Figure A 2 hrs Day : Cell seeding Day 9: +ng/ml TNF-α Day 9: Fixation B Merge Nestin PH-3 Hoechst Acute TNF-α Control
Supplementary Figure 1. Therapeutic efficacy of combined NDV and anti-ctla-4 therapy is attenuated with a larger tumor challenge.
 Supplementary Figure 1. Therapeutic efficacy of combined NDV and anti-ctla-4 therapy is attenuated with a larger tumor challenge. Bilateral flank B16-F10 tumors were established as described and the animals
Supplementary Figure 1. Therapeutic efficacy of combined NDV and anti-ctla-4 therapy is attenuated with a larger tumor challenge. Bilateral flank B16-F10 tumors were established as described and the animals
Aluminum Electrolytic Capacitors
 Aluminum Electrolytic Capacitors Snap-In, Mini., 105 C, High Ripple APS TS-NH ECE-S (G) Series: TS-NH Features Long life: 105 C 2,000 hours; high ripple current handling ability Wide CV value range (47
Aluminum Electrolytic Capacitors Snap-In, Mini., 105 C, High Ripple APS TS-NH ECE-S (G) Series: TS-NH Features Long life: 105 C 2,000 hours; high ripple current handling ability Wide CV value range (47
Supplementary Materials for. Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides
 Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Μαρία Κατσιφοδήμου. Ο ρόλος της έκκρισης HLA-G από τα ανθρώπινα έμβρυα στην επιτυχία της εξωσωματικής γονιμοποίησης. Μεταπτυχιακή Διπλωματική Εργασία
 ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΣΧΟΛΗ ΘΕΤΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΤΜΗΜΑ ΒΙΟΛΟΓΙΑΣ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΚΑΤΕΥΘΥΝΣΗ: ΕΦΑΡΜΟΣΜΕΝΗ ΓΕΝΕΤΙΚΗ ΚΑΙ ΒΙΟΤΕΧΝΟΛΟΓΙΑ Ο ρόλος της έκκρισης HLA-G από τα ανθρώπινα
ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΣΧΟΛΗ ΘΕΤΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΤΜΗΜΑ ΒΙΟΛΟΓΙΑΣ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΚΑΤΕΥΘΥΝΣΗ: ΕΦΑΡΜΟΣΜΕΝΗ ΓΕΝΕΤΙΚΗ ΚΑΙ ΒΙΟΤΕΧΝΟΛΟΓΙΑ Ο ρόλος της έκκρισης HLA-G από τα ανθρώπινα
Supporting Information
 Supporting Information rigin of the Regio- and Stereoselectivity of Allylic Substitution of rganocopper Reagents Naohiko Yoshikai, Song-Lin Zhang, and Eiichi Nakamura* Department of Chemistry, The University
Supporting Information rigin of the Regio- and Stereoselectivity of Allylic Substitution of rganocopper Reagents Naohiko Yoshikai, Song-Lin Zhang, and Eiichi Nakamura* Department of Chemistry, The University
Math 6 SL Probability Distributions Practice Test Mark Scheme
 Math 6 SL Probability Distributions Practice Test Mark Scheme. (a) Note: Award A for vertical line to right of mean, A for shading to right of their vertical line. AA N (b) evidence of recognizing symmetry
Math 6 SL Probability Distributions Practice Test Mark Scheme. (a) Note: Award A for vertical line to right of mean, A for shading to right of their vertical line. AA N (b) evidence of recognizing symmetry
APPENDICES APPENDIX A. STATISTICAL TABLES AND CHARTS 651 APPENDIX B. BIBLIOGRAPHY 677 APPENDIX C. ANSWERS TO SELECTED EXERCISES 679
 APPENDICES APPENDIX A. STATISTICAL TABLES AND CHARTS 1 Table I Summary of Common Probability Distributions 2 Table II Cumulative Standard Normal Distribution Table III Percentage Points, 2 of the Chi-Squared
APPENDICES APPENDIX A. STATISTICAL TABLES AND CHARTS 1 Table I Summary of Common Probability Distributions 2 Table II Cumulative Standard Normal Distribution Table III Percentage Points, 2 of the Chi-Squared
Si + Al Mg Fe + Mn +Ni Ca rim Ca p.f.u
 .6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
.6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
Comparison of carbon-sulfur and carbon-amine bond in therapeutic drug: -S-aromatic heterocyclic podophyllum derivatives display antitumor activity
 Comparison of carbon-sulfur and carbon-amine bond in therapeutic drug: -S-aromatic heterocyclic podophyllum derivatives display antitumor activity Jian-Long Li 1,a, Wei Zhao 1,a, Chen Zhou 1,a, Ya-Xuan
Comparison of carbon-sulfur and carbon-amine bond in therapeutic drug: -S-aromatic heterocyclic podophyllum derivatives display antitumor activity Jian-Long Li 1,a, Wei Zhao 1,a, Chen Zhou 1,a, Ya-Xuan
Synthesis and Biological Evaluation of Novel Acyclic and Cyclic Glyoxamide derivatives as Bacterial Quorum Sensing and Biofilm Inhibitors
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Synthesis and Biological Evaluation of Novel Acyclic and Cyclic Glyoxamide
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Synthesis and Biological Evaluation of Novel Acyclic and Cyclic Glyoxamide
Πολιτιστικό Προφίλ Δήμου Κορυδαλλού
 Πρόγραμμα «Είμαστε Όλοι Πολίτες» του Χρηματοδοτικού Μηχανισμού του Ευρωπαϊκού Οικονομικού Χώρου (ΕΟΧ) 2009-2014 για τις Μη Κυβερνητικές Οργανώσεις (ΜΚΟ) Πρόσκληση «Προαγωγή των Δημοκρατικών Αξιών, συμπεριλαμβανομένων
Πρόγραμμα «Είμαστε Όλοι Πολίτες» του Χρηματοδοτικού Μηχανισμού του Ευρωπαϊκού Οικονομικού Χώρου (ΕΟΧ) 2009-2014 για τις Μη Κυβερνητικές Οργανώσεις (ΜΚΟ) Πρόσκληση «Προαγωγή των Δημοκρατικών Αξιών, συμπεριλαμβανομένων
SUPPLEMENTARY MATERIAL
 10.1071/CH16014_AC The Authors 2016 Australian Journal of Chemistry 2016, 69(9), 1049-1053 SUPPLEMENTARY MATERIAL A Green Approach for the Synthesis of Novel 7,11-Dihydro-6H-chromeno[3,4- e]isoxazolo[5,4-b]pyridin-6-one
10.1071/CH16014_AC The Authors 2016 Australian Journal of Chemistry 2016, 69(9), 1049-1053 SUPPLEMENTARY MATERIAL A Green Approach for the Synthesis of Novel 7,11-Dihydro-6H-chromeno[3,4- e]isoxazolo[5,4-b]pyridin-6-one
a 2,5 b 2,5 upplemental Figure 1 IL4 (4h) in Ja18-/- mice IFN-γ (16h) in Ja18-/- mice 1,5 1,5 ng/ml ng/ml 0,5 0,5 4ClPh PyrC 4ClPh PyrC
 a 2,5 IL4 (4h) in Ja18-/- mice b 2,5 IFN-γ (16h) in Ja18-/- mice 2 2 ng/ml 1,5 ng/ml 1,5 1 1,5,5 4ClPh NC PyrC 4ClPh NC PyrC upplemental Figure 1 a b c PyrC-α-GalCer NU-α-GalCer α-galcer CD4 (PE) MFI CD4
a 2,5 IL4 (4h) in Ja18-/- mice b 2,5 IFN-γ (16h) in Ja18-/- mice 2 2 ng/ml 1,5 ng/ml 1,5 1 1,5,5 4ClPh NC PyrC 4ClPh NC PyrC upplemental Figure 1 a b c PyrC-α-GalCer NU-α-GalCer α-galcer CD4 (PE) MFI CD4
Aluminum Electrolytic Capacitors (Large Can Type)
 Aluminum Electrolytic Capacitors (Large Can Type) Snap-In, 85 C TS-U ECE-S (U) Series: TS-U Features General purpose Wide CV value range (33 ~ 47,000 µf/16 4V) Various case sizes Top vent construction
Aluminum Electrolytic Capacitors (Large Can Type) Snap-In, 85 C TS-U ECE-S (U) Series: TS-U Features General purpose Wide CV value range (33 ~ 47,000 µf/16 4V) Various case sizes Top vent construction
BD OptEIA ELISA Sets ELISA
 BD OptEIA ELISA Sets ELISA BD OptEIA ELISA Sets ELISA CaptureDetection Detection 9620 170,000 ELISAEnzymed-Linked ImmunoSorbent Assay CaptureDetection BD OptEIA ELISA Set 2 BD OptEIA ELISA Set BD OptEIA
BD OptEIA ELISA Sets ELISA BD OptEIA ELISA Sets ELISA CaptureDetection Detection 9620 170,000 ELISAEnzymed-Linked ImmunoSorbent Assay CaptureDetection BD OptEIA ELISA Set 2 BD OptEIA ELISA Set BD OptEIA
ΕΠΙΔΡΑΣΗ ΤΗΣ ΧΡΗΣΗΣ ΕΛΑΙΟΠΛΑΚΟΥΝΤΑ ΣΤΗΝ ΔΙΑΤΡΟΦΗ ΤΩΝ ΑΙΓΩΝ ΔΑΜΑΣΚΟΥ ΩΣ ΠΡΟΣ ΤΗΝ ΠΟΣΟΤΗΤΑ ΚΑΙ ΠΟΙΟΤΗΤΑ ΤΟΥ ΠΑΡΑΓΟΜΕΝΟΥ ΓΑΛΑΚΤΟΣ
 Σχολή Γεωπονικών Επιστημών και Διαχείρισης Περιβάλλοντος Πτυχιακή εργασία ΕΠΙΔΡΑΣΗ ΤΗΣ ΧΡΗΣΗΣ ΕΛΑΙΟΠΛΑΚΟΥΝΤΑ ΣΤΗΝ ΔΙΑΤΡΟΦΗ ΤΩΝ ΑΙΓΩΝ ΔΑΜΑΣΚΟΥ ΩΣ ΠΡΟΣ ΤΗΝ ΠΟΣΟΤΗΤΑ ΚΑΙ ΠΟΙΟΤΗΤΑ ΤΟΥ ΠΑΡΑΓΟΜΕΝΟΥ ΓΑΛΑΚΤΟΣ
Σχολή Γεωπονικών Επιστημών και Διαχείρισης Περιβάλλοντος Πτυχιακή εργασία ΕΠΙΔΡΑΣΗ ΤΗΣ ΧΡΗΣΗΣ ΕΛΑΙΟΠΛΑΚΟΥΝΤΑ ΣΤΗΝ ΔΙΑΤΡΟΦΗ ΤΩΝ ΑΙΓΩΝ ΔΑΜΑΣΚΟΥ ΩΣ ΠΡΟΣ ΤΗΝ ΠΟΣΟΤΗΤΑ ΚΑΙ ΠΟΙΟΤΗΤΑ ΤΟΥ ΠΑΡΑΓΟΜΕΝΟΥ ΓΑΛΑΚΤΟΣ
Enantioselective Organocatalytic Michael Addition of Isorhodanines. to α, β-unsaturated Aldehydes
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Supplemental Table S1. Tumor specific networks are enriched with somatically mutated genes (taken from the database COSMIC)
 Additional file 1 Supplemental Table S1. Tumor specific networks are enriched with somatically mutated genes (taken from the database COSMIC) COSMIC genes in tumor network COSMIC genes not in tumor network
Additional file 1 Supplemental Table S1. Tumor specific networks are enriched with somatically mutated genes (taken from the database COSMIC) COSMIC genes in tumor network COSMIC genes not in tumor network
the total number of electrons passing through the lamp.
 1. A 12 V 36 W lamp is lit to normal brightness using a 12 V car battery of negligible internal resistance. The lamp is switched on for one hour (3600 s). For the time of 1 hour, calculate (i) the energy
1. A 12 V 36 W lamp is lit to normal brightness using a 12 V car battery of negligible internal resistance. The lamp is switched on for one hour (3600 s). For the time of 1 hour, calculate (i) the energy
 CSAE WPS/2009-06 Figure 1: Cut Flower Exports from Kenya, 1995-2007 Table 1: Firms in Areas with and w/out Conflict Panel A - Export Records Variable Observations Mean in No-Conflict
CSAE WPS/2009-06 Figure 1: Cut Flower Exports from Kenya, 1995-2007 Table 1: Firms in Areas with and w/out Conflict Panel A - Export Records Variable Observations Mean in No-Conflict
A Bonus-Malus System as a Markov Set-Chain. Małgorzata Niemiec Warsaw School of Economics Institute of Econometrics
 A Bonus-Malus System as a Markov Set-Chain Małgorzata Niemiec Warsaw School of Economics Institute of Econometrics Contents 1. Markov set-chain 2. Model of bonus-malus system 3. Example 4. Conclusions
A Bonus-Malus System as a Markov Set-Chain Małgorzata Niemiec Warsaw School of Economics Institute of Econometrics Contents 1. Markov set-chain 2. Model of bonus-malus system 3. Example 4. Conclusions
«Συντήρηση αχλαδιών σε νερό. υπό την παρουσία σπόρων σιναπιού (Sinapis arvensis).»
 ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΓΕΩΠΟΝΙΚΗ ΣΧΟΛΗ ΤΟΜΕΑΣ ΕΠΙΣΤΗΜΗΣ & ΤΕΧΝΟΛΟΓΙΑΣ ΤΡΟΦΙΜΩΝ ΕΛΕΝΗ Π. ΠΑΠΑΤΣΑΡΟΥΧΑ Πτυχιούχος Τεχνολόγος Τροφίμων της Γεωπονικής Σχολής (Α.Π.Θ.) «Συντήρηση αχλαδιών σε
ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΓΕΩΠΟΝΙΚΗ ΣΧΟΛΗ ΤΟΜΕΑΣ ΕΠΙΣΤΗΜΗΣ & ΤΕΧΝΟΛΟΓΙΑΣ ΤΡΟΦΙΜΩΝ ΕΛΕΝΗ Π. ΠΑΠΑΤΣΑΡΟΥΧΑ Πτυχιούχος Τεχνολόγος Τροφίμων της Γεωπονικής Σχολής (Α.Π.Θ.) «Συντήρηση αχλαδιών σε
Supporting Information. Experimental section
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
ΚΥΠΡΙΑΚΗ ΕΤΑΙΡΕΙΑ ΠΛΗΡΟΦΟΡΙΚΗΣ CYPRUS COMPUTER SOCIETY ΠΑΓΚΥΠΡΙΟΣ ΜΑΘΗΤΙΚΟΣ ΔΙΑΓΩΝΙΣΜΟΣ ΠΛΗΡΟΦΟΡΙΚΗΣ 19/5/2007
 Οδηγίες: Να απαντηθούν όλες οι ερωτήσεις. Αν κάπου κάνετε κάποιες υποθέσεις να αναφερθούν στη σχετική ερώτηση. Όλα τα αρχεία που αναφέρονται στα προβλήματα βρίσκονται στον ίδιο φάκελο με το εκτελέσιμο
Οδηγίες: Να απαντηθούν όλες οι ερωτήσεις. Αν κάπου κάνετε κάποιες υποθέσεις να αναφερθούν στη σχετική ερώτηση. Όλα τα αρχεία που αναφέρονται στα προβλήματα βρίσκονται στον ίδιο φάκελο με το εκτελέσιμο
ΕΙΣΑΓΩΓΗ ΣΤΗ ΣΤΑΤΙΣΤΙΚΗ ΑΝΑΛΥΣΗ
 ΕΙΣΑΓΩΓΗ ΣΤΗ ΣΤΑΤΙΣΤΙΚΗ ΑΝΑΛΥΣΗ ΕΛΕΝΑ ΦΛΟΚΑ Επίκουρος Καθηγήτρια Τµήµα Φυσικής, Τοµέας Φυσικής Περιβάλλοντος- Μετεωρολογίας ΓΕΝΙΚΟΙ ΟΡΙΣΜΟΙ Πληθυσµός Σύνολο ατόµων ή αντικειµένων στα οποία αναφέρονται
ΕΙΣΑΓΩΓΗ ΣΤΗ ΣΤΑΤΙΣΤΙΚΗ ΑΝΑΛΥΣΗ ΕΛΕΝΑ ΦΛΟΚΑ Επίκουρος Καθηγήτρια Τµήµα Φυσικής, Τοµέας Φυσικής Περιβάλλοντος- Μετεωρολογίας ΓΕΝΙΚΟΙ ΟΡΙΣΜΟΙ Πληθυσµός Σύνολο ατόµων ή αντικειµένων στα οποία αναφέρονται
; +302 ; +313; +320,.
 1.,,*+, - +./ +/2 +, -. ; +, - +* cm : Key words: snow-water content, surface soil, snow type, water permeability, water retention +,**. +,,**/.. +30- +302 ; +302 ; +313; +320,. + *+, *2// + -.*, **. **+.,
1.,,*+, - +./ +/2 +, -. ; +, - +* cm : Key words: snow-water content, surface soil, snow type, water permeability, water retention +,**. +,,**/.. +30- +302 ; +302 ; +313; +320,. + *+, *2// + -.*, **. **+.,
Supporting Information
 Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
EE512: Error Control Coding
 EE512: Error Control Coding Solution for Assignment on Finite Fields February 16, 2007 1. (a) Addition and Multiplication tables for GF (5) and GF (7) are shown in Tables 1 and 2. + 0 1 2 3 4 0 0 1 2 3
EE512: Error Control Coding Solution for Assignment on Finite Fields February 16, 2007 1. (a) Addition and Multiplication tables for GF (5) and GF (7) are shown in Tables 1 and 2. + 0 1 2 3 4 0 0 1 2 3
Cite as: Pol Antras, course materials for International Economics I, Spring MIT OpenCourseWare (http://ocw.mit.edu/), Massachusetts
 / / σ/σ σ/σ θ θ θ θ y 1 0.75 0.5 0.25 0 0 0.5 1 1.5 2 θ θ θ x θ θ Φ θ Φ θ Φ π θ /Φ γφ /θ σ θ π θ Φ θ θ Φ θ θ θ θ σ θ / Φ θ θ / Φ / θ / θ Normalized import share: (Xni / Xn) / (XII / XI) 1 0.1 0.01 0.001
/ / σ/σ σ/σ θ θ θ θ y 1 0.75 0.5 0.25 0 0 0.5 1 1.5 2 θ θ θ x θ θ Φ θ Φ θ Φ π θ /Φ γφ /θ σ θ π θ Φ θ θ Φ θ θ θ θ σ θ / Φ θ θ / Φ / θ / θ Normalized import share: (Xni / Xn) / (XII / XI) 1 0.1 0.01 0.001
ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΓΕΩΤΕΧΝΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΚΑΙ ΔΙΑΧΕΙΡΙΣΗΣ ΠΕΡΙΒΑΛΛΟΝΤΟΣ. Πτυχιακή εργασία
 ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΓΕΩΤΕΧΝΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΚΑΙ ΔΙΑΧΕΙΡΙΣΗΣ ΠΕΡΙΒΑΛΛΟΝΤΟΣ Πτυχιακή εργασία ΥΔΡΟΠΟΝΙΚΗ ΚΑΛΛΙΕΡΓΕΙΑ ΔΥΟΣΜΟΥ ΣΕ ΔΙΑΦΟΡΕΤΙΚΑ ΘΡΕΠΤΙΚΑ ΔΙΑΛΥΜΑΤΑ ΕΡΑΤΩ ΝΙΚΟΛΑΪΔΟΥ Λεμεσός 2014
ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΓΕΩΤΕΧΝΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΚΑΙ ΔΙΑΧΕΙΡΙΣΗΣ ΠΕΡΙΒΑΛΛΟΝΤΟΣ Πτυχιακή εργασία ΥΔΡΟΠΟΝΙΚΗ ΚΑΛΛΙΕΡΓΕΙΑ ΔΥΟΣΜΟΥ ΣΕ ΔΙΑΦΟΡΕΤΙΚΑ ΘΡΕΠΤΙΚΑ ΔΙΑΛΥΜΑΤΑ ΕΡΑΤΩ ΝΙΚΟΛΑΪΔΟΥ Λεμεσός 2014
Nowhere-zero flows Let be a digraph, Abelian group. A Γ-circulation in is a mapping : such that, where, and : tail in X, head in
 Nowhere-zero flows Let be a digraph, Abelian group. A Γ-circulation in is a mapping : such that, where, and : tail in X, head in : tail in X, head in A nowhere-zero Γ-flow is a Γ-circulation such that
Nowhere-zero flows Let be a digraph, Abelian group. A Γ-circulation in is a mapping : such that, where, and : tail in X, head in : tail in X, head in A nowhere-zero Γ-flow is a Γ-circulation such that
Nickel and Platinum PCP Pincer Complexes Incorporating an Acyclic Diaminoalkyl Central Moiety Connecting Imidazole or Pyrazole Rings
 ickel and Platinum PCP Pincer Complexes Incorporating an Acyclic Diaminoalkyl Central Moiety Connecting Imidazole or Pyrazole Rings Braulio M. Puerta Lombardi, Rudy M. Braun, Chris Gendy, Chia Yun Chang,
ickel and Platinum PCP Pincer Complexes Incorporating an Acyclic Diaminoalkyl Central Moiety Connecting Imidazole or Pyrazole Rings Braulio M. Puerta Lombardi, Rudy M. Braun, Chris Gendy, Chia Yun Chang,
Mean bond enthalpy Standard enthalpy of formation Bond N H N N N N H O O O
 Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΣΤΙΣ «ΚΛΙΝΙΚΕΣ ΚΑΙ ΚΛΙΝΙΚΟΕΡΓΑΣΤΗΡΙΑΚΕΣ ΙΑΤΡΙΚΕΣ ΕΙΔΙΚΟΤΗΤΕΣ»
 ΠΑΝΕΠΙΣΤΗΜΙΟ ΠΑΤΡΩΝ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ ΤΜΗΜΑ ΙΑΤΡΙΚΗΣ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΣΤΙΣ «ΚΛΙΝΙΚΕΣ ΚΑΙ ΚΛΙΝΙΚΟΕΡΓΑΣΤΗΡΙΑΚΕΣ ΙΑΤΡΙΚΕΣ ΕΙΔΙΚΟΤΗΤΕΣ» ΑΡΝΗΤΙΚΗ ΡΥΘΜΙΣΗ ΤΗΣ ΜΕΤΑΒΙΒΑΣΗΣ ΤΟΥ ΣΗΜΑΤΟΣ ΤΗΣ
ΠΑΝΕΠΙΣΤΗΜΙΟ ΠΑΤΡΩΝ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ ΤΜΗΜΑ ΙΑΤΡΙΚΗΣ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΣΤΙΣ «ΚΛΙΝΙΚΕΣ ΚΑΙ ΚΛΙΝΙΚΟΕΡΓΑΣΤΗΡΙΑΚΕΣ ΙΑΤΡΙΚΕΣ ΕΙΔΙΚΟΤΗΤΕΣ» ΑΡΝΗΤΙΚΗ ΡΥΘΜΙΣΗ ΤΗΣ ΜΕΤΑΒΙΒΑΣΗΣ ΤΟΥ ΣΗΜΑΤΟΣ ΤΗΣ
Electronic Supporting Information. 3-Aminothiophenecarboxylic acid (3-Atc)-induced folding in peptides
 Electronic Supplementary Material (ESI for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 Electronic Supporting Information
Electronic Supplementary Material (ESI for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 Electronic Supporting Information
FORMULAS FOR STATISTICS 1
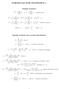 FORMULAS FOR STATISTICS 1 X = 1 n Sample statistics X i or x = 1 n x i (sample mean) S 2 = 1 n 1 s 2 = 1 n 1 (X i X) 2 = 1 n 1 (x i x) 2 = 1 n 1 Xi 2 n n 1 X 2 x 2 i n n 1 x 2 or (sample variance) E(X)
FORMULAS FOR STATISTICS 1 X = 1 n Sample statistics X i or x = 1 n x i (sample mean) S 2 = 1 n 1 s 2 = 1 n 1 (X i X) 2 = 1 n 1 (x i x) 2 = 1 n 1 Xi 2 n n 1 X 2 x 2 i n n 1 x 2 or (sample variance) E(X)
SYNkinase Catalogue. September Kinase Inhibitor Library Arrays. 1 Multi-Target Arrays. 2 Pathway and Family Specific Arrays
 SYNkinase Catalogue September 2014 Kinase Inhibitor Library Arrays Pre-made Inhibitor Array Options 1 Multi-Target Arrays 2 Pathway and Family Specific Arrays 9 Custom Array Options 16 Abl Kinase Inhibitor
SYNkinase Catalogue September 2014 Kinase Inhibitor Library Arrays Pre-made Inhibitor Array Options 1 Multi-Target Arrays 2 Pathway and Family Specific Arrays 9 Custom Array Options 16 Abl Kinase Inhibitor
ΜΕΛΕΤΗ ΤΗΣ ΗΛΕΚΤΡΟΝΙΚΗΣ ΣΥΝΤΑΓΟΓΡΑΦΗΣΗΣ ΚΑΙ Η ΔΙΕΡΕΥΝΗΣΗ ΤΗΣ ΕΦΑΡΜΟΓΗΣ ΤΗΣ ΣΤΗΝ ΕΛΛΑΔΑ: Ο.Α.Ε.Ε. ΠΕΡΙΦΕΡΕΙΑ ΠΕΛΟΠΟΝΝΗΣΟΥ ΚΑΣΚΑΦΕΤΟΥ ΣΩΤΗΡΙΑ
 ΠΑΝΕΠΙΣΤΗΜΙΟ ΠΕΙΡΑΙΩΣ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΔΙΟΙΚΗΣΗ ΤΗΣ ΥΓΕΙΑΣ ΤΕΙ ΠΕΙΡΑΙΑ ΜΕΛΕΤΗ ΤΗΣ ΗΛΕΚΤΡΟΝΙΚΗΣ ΣΥΝΤΑΓΟΓΡΑΦΗΣΗΣ ΚΑΙ Η ΔΙΕΡΕΥΝΗΣΗ ΤΗΣ ΕΦΑΡΜΟΓΗΣ ΤΗΣ ΣΤΗΝ ΕΛΛΑΔΑ: Ο.Α.Ε.Ε. ΠΕΡΙΦΕΡΕΙΑ ΠΕΛΟΠΟΝΝΗΣΟΥ
ΠΑΝΕΠΙΣΤΗΜΙΟ ΠΕΙΡΑΙΩΣ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΔΙΟΙΚΗΣΗ ΤΗΣ ΥΓΕΙΑΣ ΤΕΙ ΠΕΙΡΑΙΑ ΜΕΛΕΤΗ ΤΗΣ ΗΛΕΚΤΡΟΝΙΚΗΣ ΣΥΝΤΑΓΟΓΡΑΦΗΣΗΣ ΚΑΙ Η ΔΙΕΡΕΥΝΗΣΗ ΤΗΣ ΕΦΑΡΜΟΓΗΣ ΤΗΣ ΣΤΗΝ ΕΛΛΑΔΑ: Ο.Α.Ε.Ε. ΠΕΡΙΦΕΡΕΙΑ ΠΕΛΟΠΟΝΝΗΣΟΥ
[1] P Q. Fig. 3.1
![[1] P Q. Fig. 3.1 [1] P Q. Fig. 3.1](/thumbs/79/80362156.jpg) 1 (a) Define resistance....... [1] (b) The smallest conductor within a computer processing chip can be represented as a rectangular block that is one atom high, four atoms wide and twenty atoms long. One
1 (a) Define resistance....... [1] (b) The smallest conductor within a computer processing chip can be represented as a rectangular block that is one atom high, four atoms wide and twenty atoms long. One
Jesse Maassen and Mark Lundstrom Purdue University November 25, 2013
 Notes on Average Scattering imes and Hall Factors Jesse Maassen and Mar Lundstrom Purdue University November 5, 13 I. Introduction 1 II. Solution of the BE 1 III. Exercises: Woring out average scattering
Notes on Average Scattering imes and Hall Factors Jesse Maassen and Mar Lundstrom Purdue University November 5, 13 I. Introduction 1 II. Solution of the BE 1 III. Exercises: Woring out average scattering
Supplementary figures
 A Supplementary figures a) DMT.BG2 0.87 0.87 0.72 20 40 60 80 100 DMT.EG2 0.93 0.85 20 40 60 80 EMT.MG3 0.85 0 20 40 60 80 20 40 60 80 100 20 40 60 80 100 20 40 60 80 EMT.G6 DMT/EMT b) EG2 0.92 0.85 5
A Supplementary figures a) DMT.BG2 0.87 0.87 0.72 20 40 60 80 100 DMT.EG2 0.93 0.85 20 40 60 80 EMT.MG3 0.85 0 20 40 60 80 20 40 60 80 100 20 40 60 80 100 20 40 60 80 EMT.G6 DMT/EMT b) EG2 0.92 0.85 5
IL - 13 /IL - 18 ELISA PCR RT - PCR. IL - 13 IL - 18 mrna. 13 IL - 18 mrna IL - 13 /IL Th1 /Th2
 344 IL - 13 /IL - 18 1 2 1 2 1 2 1 2 1 2 3 1 2 13 18 IL - 13 /IL - 18 10% / OVA /AL OH 3 5% 16 ~ 43 d 44 d ELISA BALF IL - 13 IL - 18 PCR RT - PCR IL - 13 IL - 18 mrna IL - 13 mrna 0. 01 IL - 18 mrna 0.
344 IL - 13 /IL - 18 1 2 1 2 1 2 1 2 1 2 3 1 2 13 18 IL - 13 /IL - 18 10% / OVA /AL OH 3 5% 16 ~ 43 d 44 d ELISA BALF IL - 13 IL - 18 PCR RT - PCR IL - 13 IL - 18 mrna IL - 13 mrna 0. 01 IL - 18 mrna 0.
VBA Microsoft Excel. J. Comput. Chem. Jpn., Vol. 5, No. 1, pp (2006)
 J. Comput. Chem. Jpn., Vol. 5, No. 1, pp. 29 38 (2006) Microsoft Excel, 184-8588 2-24-16 e-mail: yosimura@cc.tuat.ac.jp (Received: July 28, 2005; Accepted for publication: October 24, 2005; Published on
J. Comput. Chem. Jpn., Vol. 5, No. 1, pp. 29 38 (2006) Microsoft Excel, 184-8588 2-24-16 e-mail: yosimura@cc.tuat.ac.jp (Received: July 28, 2005; Accepted for publication: October 24, 2005; Published on
Διδακτορική Διατριβή
 ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ Διδακτορική Διατριβή Η ΕΠΙΔΡΑΣΗ ΕΚΠΑΙΔΕΥΤΙΚΗΣ ΠΑΡΕΜΒΑΣΗΣ ΥΠΟΒΟΗΘΟΥΜΕΝΗΣ ΑΠΟ ΥΠΟΛΟΓΙΣΤΗ ΣΤΗΝ ΠΟΙΟΤΗΤΑ ΖΩΗΣ ΚΑΙ ΣΤΗΝ ΑΥΤΟΦΡΟΝΤΙΔΑ ΑΣΘΕΝΩΝ ΜΕ ΚΑΡΔΙΑΚΗ
ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ Διδακτορική Διατριβή Η ΕΠΙΔΡΑΣΗ ΕΚΠΑΙΔΕΥΤΙΚΗΣ ΠΑΡΕΜΒΑΣΗΣ ΥΠΟΒΟΗΘΟΥΜΕΝΗΣ ΑΠΟ ΥΠΟΛΟΓΙΣΤΗ ΣΤΗΝ ΠΟΙΟΤΗΤΑ ΖΩΗΣ ΚΑΙ ΣΤΗΝ ΑΥΤΟΦΡΟΝΤΙΔΑ ΑΣΘΕΝΩΝ ΜΕ ΚΑΡΔΙΑΚΗ
Contents Part I Psychoneuroimmunology and Systems Biology Mechanisms 1 From Psychoneuroimmunology to Personalized, Systems, and Dynamical Medicine
 Contents Part I Psychoneuroimmunology and Systems Biology Mechanisms 1 From Psychoneuroimmunology to Personalized, Systems, and Dynamical Medicine... 3 1.1 Psychoneuroimmunology (PNI) and Systems Biology...
Contents Part I Psychoneuroimmunology and Systems Biology Mechanisms 1 From Psychoneuroimmunology to Personalized, Systems, and Dynamical Medicine... 3 1.1 Psychoneuroimmunology (PNI) and Systems Biology...
Homework 3 Solutions
 Homework 3 Solutions Igor Yanovsky (Math 151A TA) Problem 1: Compute the absolute error and relative error in approximations of p by p. (Use calculator!) a) p π, p 22/7; b) p π, p 3.141. Solution: For
Homework 3 Solutions Igor Yanovsky (Math 151A TA) Problem 1: Compute the absolute error and relative error in approximations of p by p. (Use calculator!) a) p π, p 22/7; b) p π, p 3.141. Solution: For
Does anemia contribute to end-organ dysfunction in ICU patients Statistical Analysis
 Does anemia contribute to end-organ dysfunction in ICU patients Statistical Analysis Xue Han, MPH and Matt Shotwell, PhD Department of Biostatistics Vanderbilt University School of Medicine March 14, 2014
Does anemia contribute to end-organ dysfunction in ICU patients Statistical Analysis Xue Han, MPH and Matt Shotwell, PhD Department of Biostatistics Vanderbilt University School of Medicine March 14, 2014
High mobility group 1 HMG1
 Vol. 29, pp.705 ~ 711, 2001 High mobility group 1 HMG1 13 12 20 anti-neutrophil cytoplasmic antibodies, ANCA ANCA 1982 Davies 1980 1 high mobility group HMG1 HMG2 30 kd high mobility group HMGHMG HMG1
Vol. 29, pp.705 ~ 711, 2001 High mobility group 1 HMG1 13 12 20 anti-neutrophil cytoplasmic antibodies, ANCA ANCA 1982 Davies 1980 1 high mobility group HMG1 HMG2 30 kd high mobility group HMGHMG HMG1
ΠΑΝΔΠΗΣΖΜΗΟ ΠΑΣΡΩΝ ΣΜΖΜΑ ΖΛΔΚΣΡΟΛΟΓΩΝ ΜΖΥΑΝΗΚΩΝ ΚΑΗ ΣΔΥΝΟΛΟΓΗΑ ΤΠΟΛΟΓΗΣΩΝ ΣΟΜΔΑ ΤΣΖΜΑΣΩΝ ΖΛΔΚΣΡΗΚΖ ΔΝΔΡΓΔΗΑ
 ΠΑΝΔΠΗΣΖΜΗΟ ΠΑΣΡΩΝ ΣΜΖΜΑ ΖΛΔΚΣΡΟΛΟΓΩΝ ΜΖΥΑΝΗΚΩΝ ΚΑΗ ΣΔΥΝΟΛΟΓΗΑ ΤΠΟΛΟΓΗΣΩΝ ΣΟΜΔΑ ΤΣΖΜΑΣΩΝ ΖΛΔΚΣΡΗΚΖ ΔΝΔΡΓΔΗΑ Γηπισκαηηθή Δξγαζία ηνπ Φνηηεηή ηνπ ηκήκαηνο Ζιεθηξνιόγσλ Μεραληθώλ θαη Σερλνινγίαο Ζιεθηξνληθώλ
ΠΑΝΔΠΗΣΖΜΗΟ ΠΑΣΡΩΝ ΣΜΖΜΑ ΖΛΔΚΣΡΟΛΟΓΩΝ ΜΖΥΑΝΗΚΩΝ ΚΑΗ ΣΔΥΝΟΛΟΓΗΑ ΤΠΟΛΟΓΗΣΩΝ ΣΟΜΔΑ ΤΣΖΜΑΣΩΝ ΖΛΔΚΣΡΗΚΖ ΔΝΔΡΓΔΗΑ Γηπισκαηηθή Δξγαζία ηνπ Φνηηεηή ηνπ ηκήκαηνο Ζιεθηξνιόγσλ Μεραληθώλ θαη Σερλνινγίαο Ζιεθηξνληθώλ
Supplementary Materials: Development of Amyloseand β-cyclodextrin-based Chiral Fluorescent Sensors Bearing Terthienyl Pendants
 S1 of S12 Supplementary Materials: Development of Amyloseand β-cyclodextrin-based Chiral Fluorescent Sensors Bearing Terthienyl Pendants Tomoyuki Ikai, Changsik Yun, Yutaka Kojima, Daisuke Suzuki, Katsuhiro
S1 of S12 Supplementary Materials: Development of Amyloseand β-cyclodextrin-based Chiral Fluorescent Sensors Bearing Terthienyl Pendants Tomoyuki Ikai, Changsik Yun, Yutaka Kojima, Daisuke Suzuki, Katsuhiro
Καρκίνος ορθού Προεγχειρητική ακτινοθεραπεία. Λουίζα Βίνη Ογκολόγος Ακτινοθεραπεύτρια Τμήμα Ακτινοθεραπείας Ιατρικό Αθηνών
 Καρκίνος ορθού Προεγχειρητική ακτινοθεραπεία Λουίζα Βίνη Ογκολόγος Ακτινοθεραπεύτρια Τμήμα Ακτινοθεραπείας Ιατρικό Αθηνών Prognostic groups in 1676 patients with T3 rectal cancer treated without preoperative
Καρκίνος ορθού Προεγχειρητική ακτινοθεραπεία Λουίζα Βίνη Ογκολόγος Ακτινοθεραπεύτρια Τμήμα Ακτινοθεραπείας Ιατρικό Αθηνών Prognostic groups in 1676 patients with T3 rectal cancer treated without preoperative
Bayesian modeling of inseparable space-time variation in disease risk
 Bayesian modeling of inseparable space-time variation in disease risk Leonhard Knorr-Held Laina Mercer Department of Statistics UW May, 013 Motivation Ohio Lung Cancer Example Lung Cancer Mortality Rates
Bayesian modeling of inseparable space-time variation in disease risk Leonhard Knorr-Held Laina Mercer Department of Statistics UW May, 013 Motivation Ohio Lung Cancer Example Lung Cancer Mortality Rates
Η Α ΕΝΟΫΠΟΦΥΣΗ: OI ΓΟΝΑ ΟΤΡΟΠΙΝΕΣ (FSH, LH) ΚΑΙ Η ΠΡΟΛΑΚΤΙΝΗ (PRL)
 Η Α ΕΝΟΫΠΟΦΥΣΗ: OI ΓΟΝΑ ΟΤΡΟΠΙΝΕΣ (FSH, LH) ΚΑΙ Η ΠΡΟΛΑΚΤΙΝΗ (PRL) Κωνσταντίνος Καλλαράς Ιατρός Παθολόγος Καθηγητής Φυσιολογίας Εργαστήριο Πειραματικής Φυσιολογίας Ιατρικής Σχολής Α.Π.Θ. FSH:ΜΒ 33000,
Η Α ΕΝΟΫΠΟΦΥΣΗ: OI ΓΟΝΑ ΟΤΡΟΠΙΝΕΣ (FSH, LH) ΚΑΙ Η ΠΡΟΛΑΚΤΙΝΗ (PRL) Κωνσταντίνος Καλλαράς Ιατρός Παθολόγος Καθηγητής Φυσιολογίας Εργαστήριο Πειραματικής Φυσιολογίας Ιατρικής Σχολής Α.Π.Θ. FSH:ΜΒ 33000,
Electronic Supplementary Information
 Electronic Supplementary Information Cucurbit[7]uril host-guest complexes of the histamine H 2 -receptor antagonist ranitidine Ruibing Wang and Donal H. Macartney* Department of Chemistry, Queen s University,
Electronic Supplementary Information Cucurbit[7]uril host-guest complexes of the histamine H 2 -receptor antagonist ranitidine Ruibing Wang and Donal H. Macartney* Department of Chemistry, Queen s University,
Supplementary Table 1. Construct List with key Biophysical Properties of the expression
 SPINE Benchmark Target ID Well Code Tag (N or C) Fusion MW (kda) Fusion pi Cleavage site Prot MW (Da) Prot pi 1 A1 OPPF 2585 N-His6 21.75 6.41 Protease 3C 19.77 5.43 2 B1 OPPF 2586 N-His6 15.6 6.27 Protease
SPINE Benchmark Target ID Well Code Tag (N or C) Fusion MW (kda) Fusion pi Cleavage site Prot MW (Da) Prot pi 1 A1 OPPF 2585 N-His6 21.75 6.41 Protease 3C 19.77 5.43 2 B1 OPPF 2586 N-His6 15.6 6.27 Protease
Supplementary Information
 Electronic upplementary Material (EI) for Photochemical & Photobiological ciences. This journal is The Royal ociety of Chemistry and wner ocieties 214 upplementary Information elective and sensitive fluorescence-shift
Electronic upplementary Material (EI) for Photochemical & Photobiological ciences. This journal is The Royal ociety of Chemistry and wner ocieties 214 upplementary Information elective and sensitive fluorescence-shift
SABiosciences PCR Array Catalog #: PAHS-021 SA+ SCF 4h stimulaton AVG(Ct) Position Unigene Refseq Symbol Description shcontr shcontr-4h shgskβ A01
 SABiosciences PCR Array Catalog #: PAHS-021 SA+ SCF 4h stimulaton AVG(Ct) Position Unigene Refseq Symbol Description shcontr shcontr-4h shgskβ A01 Hs.1274 NM_006129 BMP1 Bone morphogenetic protein 1 25.25
SABiosciences PCR Array Catalog #: PAHS-021 SA+ SCF 4h stimulaton AVG(Ct) Position Unigene Refseq Symbol Description shcontr shcontr-4h shgskβ A01 Hs.1274 NM_006129 BMP1 Bone morphogenetic protein 1 25.25
NES: normalized enrichment score (analyzed using KEGG pathway gene sets in the GSEA software); FDR:
 Supplementary table1. List of gene sets with simultaneously altered the enrichment upon SAP18 overexpression and knockdown. Gene sets enriched by SAP18 and reduced by shsap18 SAP18 against LacZ shsap18
Supplementary table1. List of gene sets with simultaneously altered the enrichment upon SAP18 overexpression and knockdown. Gene sets enriched by SAP18 and reduced by shsap18 SAP18 against LacZ shsap18
Supplementary Information 1.
 Supplementary Information 1. Fig. S1. Correlations between litter-derived-c and N (percent of initial input) and Al-/Fe- (hydr)oxides dissolved by ammonium oxalate (AO); a) 0 10 cm; b) 10 20 cm; c) 20
Supplementary Information 1. Fig. S1. Correlations between litter-derived-c and N (percent of initial input) and Al-/Fe- (hydr)oxides dissolved by ammonium oxalate (AO); a) 0 10 cm; b) 10 20 cm; c) 20
Figure 3 Three observations (Vp, Vs and density isosurfaces) intersecting in the PLF space. Solutions exist at the two indicated points.
 φ φ φ φ Figure 1 Resampling of a rock-physics model from velocity-porosity to lithology-porosity space. C i are model results for various clay contents. φ ρ ρ δ Figure 2 Bulk modulus constraint cube in
φ φ φ φ Figure 1 Resampling of a rock-physics model from velocity-porosity to lithology-porosity space. C i are model results for various clay contents. φ ρ ρ δ Figure 2 Bulk modulus constraint cube in
Τεχνικές παρασκευής ζεόλιθου ZSM-5 από τέφρα φλοιού ρυζιού με χρήση φούρνου μικροκυμάτων και τεχνικής sol-gel
 Τεχνικές παρασκευής ζεόλιθου ZSM-5 από τέφρα φλοιού ρυζιού με χρήση φούρνου μικροκυμάτων και τεχνικής sol-gel Δέσποινα Στεφοπούλου Επιβλέπων: Κωνσταντίνος Κορδάτος Στην παρούσα διπλωματική εργασία παρασκευάστηκαν
Τεχνικές παρασκευής ζεόλιθου ZSM-5 από τέφρα φλοιού ρυζιού με χρήση φούρνου μικροκυμάτων και τεχνικής sol-gel Δέσποινα Στεφοπούλου Επιβλέπων: Κωνσταντίνος Κορδάτος Στην παρούσα διπλωματική εργασία παρασκευάστηκαν
ΠΕΡΙΕΧΟΜΕΝΑ. Κεφάλαιο 1: Κεφάλαιο 2: Κεφάλαιο 3:
 4 Πρόλογος Η παρούσα διπλωµατική εργασία µε τίτλο «ιερεύνηση χωρικής κατανοµής µετεωρολογικών µεταβλητών. Εφαρµογή στον ελληνικό χώρο», ανατέθηκε από το ιεπιστηµονικό ιατµηµατικό Πρόγραµµα Μεταπτυχιακών
4 Πρόλογος Η παρούσα διπλωµατική εργασία µε τίτλο «ιερεύνηση χωρικής κατανοµής µετεωρολογικών µεταβλητών. Εφαρµογή στον ελληνικό χώρο», ανατέθηκε από το ιεπιστηµονικό ιατµηµατικό Πρόγραµµα Μεταπτυχιακών
Solutions to the Schrodinger equation atomic orbitals. Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz
 Solutions to the Schrodinger equation atomic orbitals Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz ybridization Valence Bond Approach to bonding sp 3 (Ψ 2 s + Ψ 2 px + Ψ 2 py + Ψ 2 pz) sp 2 (Ψ 2 s + Ψ 2 px + Ψ 2 py)
Solutions to the Schrodinger equation atomic orbitals Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz ybridization Valence Bond Approach to bonding sp 3 (Ψ 2 s + Ψ 2 px + Ψ 2 py + Ψ 2 pz) sp 2 (Ψ 2 s + Ψ 2 px + Ψ 2 py)
5.4 The Poisson Distribution.
 The worst thing you can do about a situation is nothing. Sr. O Shea Jackson 5.4 The Poisson Distribution. Description of the Poisson Distribution Discrete probability distribution. The random variable
The worst thing you can do about a situation is nothing. Sr. O Shea Jackson 5.4 The Poisson Distribution. Description of the Poisson Distribution Discrete probability distribution. The random variable
Supplementary information:
 Supplementary information: Monitoring changes of docosahexaenoic acid-containing lipids during the recovery process of traumatic brain injury in rat using mass spectrometry imaging Shuai Guo 1, Dan Zhou
Supplementary information: Monitoring changes of docosahexaenoic acid-containing lipids during the recovery process of traumatic brain injury in rat using mass spectrometry imaging Shuai Guo 1, Dan Zhou
Srednicki Chapter 55
 Srednicki Chapter 55 QFT Problems & Solutions A. George August 3, 03 Srednicki 55.. Use equations 55.3-55.0 and A i, A j ] = Π i, Π j ] = 0 (at equal times) to verify equations 55.-55.3. This is our third
Srednicki Chapter 55 QFT Problems & Solutions A. George August 3, 03 Srednicki 55.. Use equations 55.3-55.0 and A i, A j ] = Π i, Π j ] = 0 (at equal times) to verify equations 55.-55.3. This is our third
Πτυχιακή Εργασία Η ΠΟΙΟΤΗΤΑ ΖΩΗΣ ΤΩΝ ΑΣΘΕΝΩΝ ΜΕ ΣΤΗΘΑΓΧΗ
 ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ Πτυχιακή Εργασία Η ΠΟΙΟΤΗΤΑ ΖΩΗΣ ΤΩΝ ΑΣΘΕΝΩΝ ΜΕ ΣΤΗΘΑΓΧΗ Νικόλας Χριστοδούλου Λευκωσία, 2012 ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ
ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ Πτυχιακή Εργασία Η ΠΟΙΟΤΗΤΑ ΖΩΗΣ ΤΩΝ ΑΣΘΕΝΩΝ ΜΕ ΣΤΗΘΑΓΧΗ Νικόλας Χριστοδούλου Λευκωσία, 2012 ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ
Supplementary Information. Living Ring-Opening Polymerization of Lactones by N-Heterocyclic Olefin/Al(C 6 F 5 ) 3
 Supplementary Information Living Ring-Opening Polymerization of Lactones by N-Heterocyclic Olefin/Al(C 6 F 5 ) 3 Lewis Pairs: Structures of Intermediates, Kinetics, and Mechanism Qianyi Wang, Wuchao Zhao,
Supplementary Information Living Ring-Opening Polymerization of Lactones by N-Heterocyclic Olefin/Al(C 6 F 5 ) 3 Lewis Pairs: Structures of Intermediates, Kinetics, and Mechanism Qianyi Wang, Wuchao Zhao,
ANSWERSHEET (TOPIC = DIFFERENTIAL CALCULUS) COLLECTION #2. h 0 h h 0 h h 0 ( ) g k = g 0 + g 1 + g g 2009 =?
 Teko Classes IITJEE/AIEEE Maths by SUHAAG SIR, Bhopal, Ph (0755) 3 00 000 www.tekoclasses.com ANSWERSHEET (TOPIC DIFFERENTIAL CALCULUS) COLLECTION # Question Type A.Single Correct Type Q. (A) Sol least
Teko Classes IITJEE/AIEEE Maths by SUHAAG SIR, Bhopal, Ph (0755) 3 00 000 www.tekoclasses.com ANSWERSHEET (TOPIC DIFFERENTIAL CALCULUS) COLLECTION # Question Type A.Single Correct Type Q. (A) Sol least
ΠΩΣ ΕΠΗΡΕΑΖΕΙ Η ΜΕΡΑ ΤΗΣ ΕΒΔΟΜΑΔΑΣ ΤΙΣ ΑΠΟΔΟΣΕΙΣ ΤΩΝ ΜΕΤΟΧΩΝ ΠΡΙΝ ΚΑΙ ΜΕΤΑ ΤΗΝ ΟΙΚΟΝΟΜΙΚΗ ΚΡΙΣΗ
 Σχολή Διοίκησης και Οικονομίας Κρίστια Κυριάκου ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΔΙΟΙΚΗΣΗΣ ΚΑΙ ΟΙΚΟΝΟΜΙΑΣ ΤΜΗΜΑ ΕΜΠΟΡΙΟΥ,ΧΡΗΜΑΤΟΟΙΚΟΝΟΜΙΚΩΝ ΚΑΙ ΝΑΥΤΙΛΙΑΣ Της Κρίστιας Κυριάκου ii Έντυπο έγκρισης Παρουσιάστηκε
Σχολή Διοίκησης και Οικονομίας Κρίστια Κυριάκου ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΔΙΟΙΚΗΣΗΣ ΚΑΙ ΟΙΚΟΝΟΜΙΑΣ ΤΜΗΜΑ ΕΜΠΟΡΙΟΥ,ΧΡΗΜΑΤΟΟΙΚΟΝΟΜΙΚΩΝ ΚΑΙ ΝΑΥΤΙΛΙΑΣ Της Κρίστιας Κυριάκου ii Έντυπο έγκρισης Παρουσιάστηκε
Potential Dividers. 46 minutes. 46 marks. Page 1 of 11
 Potential Dividers 46 minutes 46 marks Page 1 of 11 Q1. In the circuit shown in the figure below, the battery, of negligible internal resistance, has an emf of 30 V. The pd across the lamp is 6.0 V and
Potential Dividers 46 minutes 46 marks Page 1 of 11 Q1. In the circuit shown in the figure below, the battery, of negligible internal resistance, has an emf of 30 V. The pd across the lamp is 6.0 V and
