Theoretical studies in the molecular Platonic solids: Pure and mixed carbon, nitrogen, phosphorus, and silicon tetrahedranes
|
|
|
- Ἀρτεμᾶς Ιωάννου
- 6 χρόνια πριν
- Προβολές:
Transcript
1 Theoretical studies in the molecular Platonic solids: Pure and mixed carbon, nitrogen, phosphorus, and silicon tetrahedranes Sierra Rayne a,* and Kaya Forest b,c a Ecologica Research, PO Box 74, 318 Rose Street, Mortlach, Saskatchewan, Canada, S0H 3E0 Department of Chemistry, Okanagan College, 583 Duncan Avenue West, Penticton, British Columbia, Canada, V2A 8E1 c Department of Environmental Engineering, Saskatchewan Institute of Applied Science and Technology, Palliser Campus, PO Box 1420, 600 6th Avenue NW, Moose Jaw, Saskatchewan, Canada, S6H 4R4 b * Corresponding author. Tel.: address: rayne.sierra@gmail.com (S. Rayne). 1
2 Abstract Calculations were conducted at the G4MP2 and G4 composite method levels of theory on the 35 potential carbon, nitrogen, silicon, and phosphorus tetrahedrane derivatives with the general form CaNbSicPdH(4-b-d) (where a+b+c+d=4). At both levels of theory, optimized electronic ground state neutral singlet gas phase ( K, 1 atm) geometries were obtained for 24 of the 35 possible C/N/Si/P tetrahedrane derivatives. Corresponding enthalpies of formation were calculated using the atomization method. Triplet state neutral tetrahedron starting geometries for all compounds either resulted in cage opening or failed to converge. Only 9 cationic and 3 anionic forms converged to stable geometries that retained the tetrahedron cage and were absent imaginary frequencies, thereby allowing the calculation of adiabatic ionization energies and electron affinities. Keywords: Tetrahedranes; Carbon, nitrogen, phosphorus, and silicon derivatives; Enthalpy of formation; Adiabatic ionization energy; Electron affinity 2
3 The Platonic solids are set of five congruent regular polygons (tetrahedron, cube, octahedron, dodecahedron, and icosahedron), each with the same number of faces meeting at all vertices (Figure 1). Tetrahedrane is the simplest Platonic solid molecule (Figure 2). Using carbon, nitrogen, silicon, and phosphorus, 35 potential tetrahedrane derivatives of the general form CaNbSicPdH(4-b-d) (where a+b+c+d=4) exist. Herein we use a compressed set notation style to denote each possible compound, omitting implied hydrogen atoms on tetravalent carbon and silicon atoms (e.g., {C,C,N,P} is C2H2NP and {N,Si,Si,Si} is Si3H3N) (Figure 3). At the G4MP2 [1] and G4 [2] levels of theory in Gaussian 09 [3], optimized electronic ground state neutral singlet (S0) gas phase geometries and thermochemical data were obtained for 24 of the 35 possible C/N/Si/P tetrahedrane derivatives (Table 1 and Figure 4). Structures where a 1 and c 1 (i.e., the organosilicon members {C,C,C,Si}, {C,C,N,Si}, {C,C,P,Si}, {C,C,Si,Si}, {C,N,N,Si}, {C,N,P,Si}, {C,N,Si,Si}, {C,P,P,Si}, {C,P,Si,Si}, and {C,Si,Si,Si}), as well as {P,Si,Si,Si}, converged to ringopened non-tetrahedrane geometries. Holme et al. [4] reported a {C,C,Si,Si} structure with geometry optimized using the Schlegel method and the 3-21G basis set, with subsequent single point calculations with the 6-31G* basis set and at the MP3/6-31G* level. We were not able to obtain converged {C,C,Si,Si} tetrahedron geometries at any of the HF/3-21G, HF/ G(d,p) [5, 6], M062X/ G(d,p) [5-7], B3LYP/ G(d,p) [5, 6, 8-10], CBS-Q//B3 [11-18], G4MP2, or G4 levels of theory. Triplet state neutral tetrahedron starting geometries for all compounds either resulted in cage opening or failed to converge. Gas phase ( K, 1 atm) enthalpies of formation (ΔfH (g)) were calculated using the atomization approach at both the G4MP2 and G4 levels of theory [19-21] (Table 1). These levels of theory should achieve effective thermochemical accuracy [1, 2, 22-28]. With the exception of phosphorus tetrahedrane (G4MP2/G4 ΔfH (g)=48.6/61.2 kj/mol), all tetrahedranes examined are expected to have substantially endothermic ΔfH (g) (from about 180 to 750 kj/mol), and no tetrahedranes considered have exothermic ΔfH (g). The mixed element tetrahedranes adopt distorted tetrahedron geometries, with increasing degrees of distortion as atomic size differences increase among the constituents (all geometries at the G4MP2 and G4 levels are provided in the Supplementary Materials). Calculations at the G4MP2 and G4 levels were also conducted on all cationic and anionic forms of these 24 C/N/P/Si tetrahedranes. Only 9 of the cations converged yielding non-cage opened tetrahedral geometries absent imaginary frequencies at the G4/G4MP2 levels ({C,N,N,N}, {C,P,P,P}, {C,N,P,P}, {N,N,P,P}, {P,P,P,P}, {P,P,Si,Si}, {N,N,P,Si}, {N,P,P,Si}, and {N,P,Si,Si}). Adiabatic ionization energies (AIEs) were calculated for these derivatives and are provided in Table 1. AIEs at the G4/G4MP2 levels are expected to be at or near thermochemical accuracy [1, 2, 24, 29]. Only three anionic structures converged at these levels of theory ({N,N,P,P}, {P,P,Si,Si}, and {N,P,Si,Si}), providing the estimated electron affinities (EAs) also given in Table 1. For the carbon and nitrogen tetrahedranes and their mixed derivatives ({C,C,C,C}, {C,C,C,N}, {C,C,N,N}, {C,N,N,N}, and {N,N,N,N}), a modest set of prior theoretical ΔfH (g) estimates exist in the literature (Table 2). The only other high-level ΔfH (g) estimates for the C/N/P/Si tetrahedranes are on the pure carbon and nitrogen members, for which our ΔfH (g) data is in excellent agreement with previous calculations using the CBS-Q ({C,C,C,C}), G2 ({C,C,C,C} and {N,N,N,N}), G3 ({N,N,N,N}), and W2 ({N,N,N,N}) methods. An experimental ΔfH (g) datapoint is only available for {P,P,P,P} (59.0 kj/mol), which is in excellent agreement with our G4 estimate (61.2 kj/mol) and reasonable agreement with our G4MP2 estimate (48.6 kj/mol). In addition, the experimental AIE for the phosphorus 3
4 tetrahedrane (9.08 to 9.34 ev) is in excellent agreement with our G4MP2 (9.16 ev) and G4 (9.20 ev) estimates. No other theoretical AIEs for these compounds appear to be available in the literature. We also conducted CBS-Q//B3 [17, 18] calculations on the pure C/N/P/Si tetrahedranes, and the corresponding atomization approach ΔfH (g) estimates are also given in Table 2, as is the ΔfH (g) estimate (765.0 kj/mol) from our W1BD [30] calculation on {N,N,N,N}. These latter calculations are also in strong agreement with our G4MP2 and G4 estimates. Acknowledgements This work was made possible by the facilities of the Western Canada Research Grid (WestGrid: project ), the Shared Hierarchical Academic Research Computing Network (SHARCNET: project sn4612), and Compute/Calcul Canada. Appendix A. Supplementary Material Supplementary data is associated with this article. 4
5 Figure Captions Figure 1. Geometrical shapes of the five Platonic solids (imagery from Figure 2. Three- and two-dimensional general representations of tetrahedrane. Figure 3. Example structures and set notation naming styles for the {C,C,N,P} and {N,Si,Si,Si} tetrahedranes. Figure 4. Three-dimensional representations of the C/N/P/Si tetrahedranes at the G4 level of theory. 5
6 Table 1. G4MP2/G4 calculated C/N/P/Si tetrahedrane gas phase ( K, 1 atm) enthalpies of formation (ΔfH g), adiabatic ionization energies (AIEs), and electron affinities (EAs). ΔfH (g) (kj/mol) AIE (ev) EA (ev) compound G4MP2 G4 G4MP2 G4 G4MP2 G4 {C,C,C,C} a {C,C,C,N} {C,C,N,N} {C,N,N,N} {C,C,C,P} {C,C,P,P} {C,P,P,P} {C,C,N,P} {C,N,N,P} {C,N,P,P} {N,N,N,N} {N,N,N,P} {N,N,P,P} {N,P,P,P} {P,P,P,P} {N,N,N,Si} {N,N,Si,Si} {N,Si,Si,Si} {P,P,P,Si} {P,P,Si,Si} {N,N,P,Si} {N,P,P,Si} {N,P,Si,Si} {Si,Si,Si,Si} a Cationic/anionic structure did not converge with a tetrahedrane geometry absent imaginary frequencies. 6
7 Table 2. Comparison of G4MP2/G4 calculated C/N/P/Si tetrahedrane gas phase ( K, 1 atm) enthalpies of formation (ΔfH g) and adiabatic ionization energies (AIEs) with prior calculations and experimental reports from the literature. compound {C,C,C,C} level of theory ΔfH g (kj/mol) BP/6-31G* SCF/6-31G* BLYP/6-31G* MINDO/ G4MP G4MP G B3LYP/aug-cc-pVDZ G CBS-Q G SCF/4-31G CBS-Q//B RMP2/6-31G* SCF/6-31G* RMP2/6-31G* SCF/DZ+D SCF/4-31G SCF/6-31G* SCF/6-31G* B3LYP/6-31G* SVWN/6-31G* SCF(MO) SCF/6-31G* SCF/6-31G AM SCF/4-21G SCF/4-31G SCF/6-31G SCF/4-21G AIE (ev) a b Ref. [31] [32] [31] [33] current work [28] [34] [35] current work [36] [28] [37] current work [32] [32] [39] [37] [32] [40] [31] [31] [41] [40] comments homodesmic reaction set using alternate atomization approach using alternate atomization approach isodesmic reaction set 2 homodesmic reaction set isodesmic reaction set 2 isodesmic reaction set isodesmic reaction set 1 isodesmic reaction set isodesmic reaction set 1 isodesmic reaction set 2 isodesmic reaction set 2 isodesmic reaction set 1 isodesmic reaction set 1 7
8 {C,C,C,N} {C,C,N,N} {C,N,N,N} {N,N,N,N} tight-binding MD G4MP2 G4 SCF/6-31G* SCF/6-31G* AM1 SCF/6-31G SCF/4-21G SCF/6-31G SCF/4-21G G4MP2 G4 SCF/6-31G* SCF/6-31G* SCF/6-31G* AM1 SCF/4-21G SCF/6-31G SCF/6-31G SCF/4-21G SCF/6-31G SCF/4-21G G4MP2 G4 SCF/6-31G* SCF/6-31G* AM1 SCF/4-21G SCF/6-31G SCF/4-21G SCF/6-31G G2 G4MP2 CBS-Q//B [42] current work current work current work current work current work current work [43] current work current work isodesmic reaction set 2 isodesmic reaction set 1 isodesmic reaction set 2 isodesmic reaction set 2 isodesmic reaction set 1 isodesmic reaction set 1 isodesmic reaction set 2 isodesmic reaction set 3 isodesmic reaction set 1 isodesmic reaction set 2 isodesmic reaction set 2 isodesmic reaction set 3 isodesmic reaction set 3 isodesmic reaction set 1 isodesmic reaction set 1 isodesmic reaction set 2 isodesmic reaction set 1 isodesmic reaction set 2 isodesmic reaction set 2 isodesmic reaction set 1 isodesmic reaction set 1 8
9 G current work W [44] W1BD current work G [45] SCF/6-31G* SCF/6-31G* AM SCF/4-21G SCF/6-31G SCF/4-21G SCF/6-31G {P,P,P,P} CBS-Q//B current work G4MP current work experimental to 9.34 [46-51] G current work {Si,Si,Si,Si} MINDO/ [52] CBS-Q//B current work G4MP current work G current work a Not available. b Stable cationic tetrahedrane geometries could not be obtained. isodesmic reaction set 2 isodesmic reaction set 1 isodesmic reaction set 2 isodesmic reaction set 2 isodesmic reaction set 1 isodesmic reaction set 1 9
10 Figures Tetrahedron Cube Octahedron Dodecahedron Icosahedron Figure 1. 10
11 Figure 2. 11
12 Figure 3. 12
13 {C,C,C,C} {C,C,C,N} {C,C,N,N} {C,N,N,N} {C,C,C,P} {C,C,P,P} {C,P,P,P} {C,C,N,P} {C,N,N,P} {C,N,P,P} {N,N,N,N} {N,N,N,P} {N,N,P,P} {N,P,P,P} {P,P,P,P} {N,N,N,Si} {N,N,Si,Si} {N,Si,Si,Si} {P,P,P,Si} {P,P,Si,Si} {N,N,P,Si} {N,P,P,Si} {N,P,Si,Si} {Si,Si,Si,Si} Figure 4. 13
14 References [1] L.A. Curtiss, P.C. Redfern, K. Raghavachari, Gaussian-4 theory using reduced order perturbation theory, J. Chem. Phys. 127 (2007) [2] L.A. Curtiss, P.C. Redfern, K. Raghavachari, Gaussian-4 theory, J. Chem. Phys. 126 (2007) [3] M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, et al., Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford, CT, [4] T.A. Holme, M.S. Gordon, S. Yabushita, M.W. Schmidt, Theoretical studies of cyclic C2Si2H4 molecules, Organometallics 3 (1984) [5] A.D. McLean, G.S. Chandler, Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11-18, J. Chem. Phys. 72 (1980) [6] K. Raghavachari, J.S. Binkley, R. Seeger, J.A. Pople, Self-consistent molecular orbital methods. 20. Basis set for correlated wave-functions, J. Chem. Phys. 72 (1980) [7] Y. Zhao, D. Truhlar, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals, Theor. Chem. Acc. 120 (2008) [8] A.D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys. 98 (1993) [9] C. Lee, W. Yang, R.G. Parr, Development of the Colle-Salvetti correlation energy formula into a functional of the electron density, Phys. Rev. B 37 (1988) [10] B. Miehlich, A. Savin, H. Stoll, H. Preuss, Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr, Chem. Phys. Lett. 157 (1989) [11] M.R. Nyden, G.A. Petersson, Complete basis set correlation energies. I. The asymptotic convergence of pair natural orbital expansions, J. Chem. Phys. 75 (1981) [12] G.A. Petersson, A. Bennett, T.G. Tensfeldt, M.A. Al-Laham, W.A. Shirley, J. Mantzaris, A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the firstrow atoms, J. Chem. Phys. 89 (1988) [13] G.A. Petersson, M.A. Al-Laham, A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms, J. Chem. Phys. 94 (1991) [14] G.A. Petersson, T.G. Tensfeldt, J.A. Montgomery, A complete basis set model chemistry. III. The complete basis set-quadratic configuration interaction family of methods, J. Chem. Phys. 94 (1991) [15] J.A. Montgomery, J.W. Ochterski, G.A. Petersson, A complete basis set model chemistry. IV. An improved atomic pair natural orbital method, J. Chem. Phys. 101 (1994) [16] J.W. Ochterski, G.A. Petersson, J.A. Montgomery, A complete basis set model chemistry. V. Extensions to six or more heavy atoms, J. Chem. Phys. 104 (1996) [17] J.A. Montgomery, M.J. Frisch, J.W. Ochterski, G.A. Petersson, A complete basis set model chemistry. VI. Use of density functional geometries and frequencies, J. Chem. Phys. 110 (1999) [18] J.A. Montgomery, M.J. Frisch, J.W. Ochterski, G.A. Petersson, A complete basis set model chemistry. VII. Use of the minimum population localization method, J. Chem. Phys. 112 (2000) [19] A. Nicolaides, A. Rauk, M.N. Glukhovtsev, L. Radom, Heats of formation from G2, G2(MP2), and G2(MP2,SVP) total energies, J. Phys. Chem. 100 (1996)
15 [20] R. Notario, O. Castano, J.L.M. Abboud, R. Gomperts, L.M. Frutos, R. Palmeiro, Organic thermochemistry at high ab initio levels. 1. A G2(MP2) and G2 study of cyclic saturated and unsaturated hydrocarbons (including aromatics), J. Org. Chem. 64 (1999) [21] J.W. Ochterski, Thermochemistry in Gaussian, Gaussian, Inc., Wallingford, CT, [22] S. Rayne, K. Forest, Performance of Gaussian-3 and Gaussian-4 level theoretical methods in estimating gas phase enthalpies of formation for representative C1 and C2 chlorofluorocarbons and hydrochlorofluorocarbons, J. Mol. Struct. (Theochem) 953 (2010) [23] S. Rayne, K. Forest, Estimated gas-phase standard state enthalpies of formation for organic compounds using the Gaussian-4 (G4) and W1BD theoretical methods, J. Chem. Eng. Data 55 (2010) [24] S. Rayne, K. Forest, Thermochemistry of mono- and disubstituted acetylenes and polyynes at the Gaussian-4 level of theory, Comp. Theor. Chem. 970 (2011) [25] S. Rayne, K. Forest, Reply to Comments by O. V. Dorofeeva on J. Chem. Eng. Data, 2010, 55, , J. Chem. Eng. Data 56 (2011) [26] S. Rayne, K. Forest, Computational note on a G4MP2 study into the gas phase enthalpies of formation and isomerization for the (CH)2n (n=1-6) isomers, J. Mol. Struct. (Theochem) 948 (2010) [27] S. Rayne, K. Forest, Gas-phase enthalpies of formation, acidities, and strain energies of the [m,n]polyprismanes (m 2; n=3-8; m n 16): A CBS-Q//B3, G4MP2, and G4 theoretical study, Theor. Chem. Acc. 127 (2010) [28] S. Rayne, K. Forest, A G4MP2 and G4 theoretical study into the thermochemical properties of explosophore substituted tetrahedranes and cubanes, Propell. Explos. Pyrot. (2011) in press. [29] S. Rayne, K. Forest, Estimated adiabatic ionization energies for organic compounds using the Gaussian-4 (G4) and W1BD theoretical methods, J. Chem. Eng. Data 56 (2011) [30] E.C. Barnes, G.A. Petersson, J.A. Montgomery, M.J. Frisch, J.M.L. Martin, Unrestricted coupled cluster and Brueckner doubles variations of W1 theory, J. Chem. Theory Comput. 5 (2009) [31] D.W. Ball, On the ΔHf values of tetrahedrane and cubane: Density functional theory calculations, J. Mol. Struct. (Theochem) 364 (1996) [32] R.L. Disch, J.M. Schulman, M.L. Sabio, Ab initio heats of formation of medium-sized hydrocarbons. 2. Use of second-order correlation energies, J. Am. Chem. Soc. 107 (1985) [33] H. Kollmar, F. Carrion, M.J.S. Dewar, R.C. Bingham, Ground states of molecules. 58. The C4H4 potential surface, J. Am. Chem. Soc. 103 (1981) [34] M.N. Glukhovtsev, S. Laiter, A. Pross, Thermochemistry of cyclobutadiene and tetrahedrane: A high-level computational study, J. Phys. Chem. 99 (1995) [35] G. Zhou, J.L. Zhang, N.B. Wong, A. Tian, Computational studies on a kind of novel energetic materials tetrahedrane and nitro derivatives, J. Mol. Struct. (Theochem) 668 (2004) [36] B.S. Jursic, Computing the heat of formation for cubane and tetrahedrane with density functional theory and complete basis set ab initio methods, J. Mol. Struct. (Theochem) 499 (2000) [37] J.M. Schulman, T.J. Venanzi, Theoretical study of the tetrahedrane molecule, J. Am. Chem. Soc. 96 (1974) I. Alkorta, J. Elguero, I. Rozas, A.T. Balaban, Theoretical studies on aza-analogs of Platonic hydrocarbons: Part II. Tetrahedrane and its azaderivatives, J. Mol. Struct. (Theochem) 208 (1990) [39] H. Kollmar, An MO theoretical study on the stability of tetrahedrane, J. Am. Chem. Soc. 102 (1980) [40] W.J. Hehre, J.A. Pople, Molecular orbital theory of the electronic structure of organic compounds. XXVI. Geometries, energies and polarities of C4 hydrocarbons, J. Am. Chem. Soc. 97 (1975)
16 [41] N.C. Baird, M.J.S. Dewar, Ground states of o-bonded molecules. II. Strain energies of cyclopropanes and cyclopropenes, J. Am. Chem. Soc. 89 (1967) [42] M.M. Maslov, K.P. Katin, On the thermal stability of tetrahedrane: Tight-binding molecular dynamics study, Chem. Phys. 387 (2011) [43] M.N. Glukhovtsev, S. Laiter, Thermochemistry of tetrazete and tetraazatetrahedrane: A high-level computational study, J. Phys. Chem. 100 (1996) [44] T.J. Lee, J.M.L. Martin, An accurate quartic force field, fundamental frequencies, and binding energy for the high energy density material TdN4, Chem. Phys. Lett. 357 (2002) [45] M.T. Nguyen, T.L. Nguyen, A.M. Mebel, R. Flammang, Azido-nitrene is probably the N4 molecule observed in mass spectrometric experiments, J. Phys. Chem. A 107 (2003) [46] J.D. Cox, D.D. Wagman, V.A. Medvedev, CODATA Key Values for Thermodynamics, Hemisphere, New York, NY, [47] M.W. Chase, NIST-JANAF themochemical tables (4th ed.), J. Phys. Chem. Ref. Data 9 (1998) [48] R.R. Hart, M.B. Robin, N.A. Kuebler, 3p orbitals, bent bonds, and the electronic spectrum of the P2 molecule, J. Chem. Phys. 42 (1965) [49] C.R. Brundle, N.A. Kuebler, M.B. Robin, H. Basch, Ionization potentials of the tetraphosphorus molecule, Inorg. Chem. 11 (1972) [50] S. Evans, P.J. Joachim, A.F. Orchard, D.W. Turner, A study of the orbital electronic structure of the P4 molecule by photoelectron spectroscopy, Int. J. Mass Spectrom. Ion Phys. 9 (1972) [51] J. Smets, P. Coppens, J. Drowart, Photoionization with mass spectrometric analysis of the tetraphosphorus molecule, Chem. Phys. 20 (1977) [52] M.J.S. Dewar, D.H. Lo, C.A. Ramsden, Ground states of molecules. XXIX. MINDO/3 calculations of compounds containing third row elements, J. Am. Chem. Soc. 97 (1975)
17 Supplementary Material Theoretical studies in the molecular Platonic solids: Pure and mixed carbon, nitrogen, phosphorus, and silicon tetrahedranes Sierra Rayne a,* and Kaya Forest b,c a Ecologica Research, PO Box 74, 318 Rose Street, Mortlach, Saskatchewan, Canada, S0H 3E0 b Department of Chemistry, Okanagan College, 583 Duncan Avenue West, Penticton, British Columbia, Canada, V2A 8E1 c Department of Environmental Engineering, Saskatchewan Institute of Applied Science and Technology, Palliser Campus, PO Box 1420, 600 6th Avenue NW, Moose Jaw, Saskatchewan, Canada, S6H 4R4 * Corresponding author. Tel.: address: rayne.sierra@gmail.com (S. Rayne). S1
18 G4MP2 archive entries for neutral singlet tetrahedranes {C,C,C,C} \\0,1\C,0, , , \C,0, , , \C,0, , , \C,0, , , \H,0, , , \H,0, , , \H,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\S tate=1-a\mp2/gtbas1= \ccsd(t)/gtbas1= \mp2/gtbas 2=0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , , , , , , , , , , \PG=C01 [X(C4H 4)]\NImag=0\\ {C,C,C,N} \\0,1\N,0, , , \C,0, , , \C,0, , , \C,0, , , \H,0, , , \H,0, , , \H,0, , , \\Version=EM6 4L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/GTMP2 LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP 2= \FreqCoord= , , , , , , , , , , , , , , , , , , , , \PG=C01 [X(C3H3N1)]\NImag=0\\ {C,C,N,N} \\0,1\N,0, , , \ N,0, , , \C,0, , , \C,0, , , \H,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2Larg exp= \mp2/gtmp2largexp= \hf/gfhfb3= \ HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , , , , \PG=C01 [X(C2H2N2)]\NImag=0\\ {C,N,N,N} \\0,1\N,0, , , \ N,0, , , \N,0, , , \C,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State =1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0. \MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \Freq Coord= , , , , S2
19 2322, , , , , , , , , , \PG=C01 [X(C1H1N3)]\NImag=0\\ {C,C,C,P} \\0,1\C,0, , , \ P,0, , , \C,0, , , \C,0, , , \H,0, , , \H,0, , , \H,0, , , \\Version=EM 64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/GTM P2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G 4MP2= \FreqCoord= , , , , , , , , , , , , , , , , , , , , \PG=C01 [X(C3H3P1)]\NImag=0\\ {C,C,P,P} \\0,1\P,0, , , \ C,0, , , \P,0, , , \C,0, , , \H,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2La rgexp= \mp2/gtmp2largexp= \hf/gfhfb3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , , , , \PG=C01 [X(C2H2P2)]\NImag=0\\ {C,P,P,P} \\0,1\P,0, , , \ P,0, , , \P,0, , , \C,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State= 1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0. \MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(C1H1P3)]\NImag=0\\ {C,C,N,P} \\0,1\C,0, , , \C,0, , , \N,0, , , \P,0, , , \H, 0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP 2LargeXP= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , ,-4 S3
20 , , , , , , \PG=C01 [X(C2H2N1P1)]\NImag=0\\ {C,N,N,P} \\0,1\C,0, , , \N,0, , , \P,0, , , \N,0, , , \H, 0, , , \\Version=EM64L-G09RevA.02\ State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBa s2=0.\mp2/gtbas3=0.\hf/gtmp2largexp= \mp2/gtmp2largexp= \hf/gfhfb3= \hf/gfhfb4= \g4mp2= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(C1H1N2P1)]\NImag=0\\ {C,N,P,P} \\0,1\C,0, , , \N,0, , , \P,0, , , \P,0, , , \H, 0, , , \\Version=EM64L-G09RevA.02\ State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBa s2=0.\mp2/gtbas3=0.\hf/gtmp2largexp= \mp2/gtmp2largexp= \hf/gfhfb3= \hf/gfhfb4= \g4mp2= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(C1H1N1P2)]\NImag=0\\ {N,N,N,N} \\0,1\N,0, , , \N,0, , , \N,0, , , \N,0, , , \\ Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTB as1= \mp2/gtbas2=0.\mp2/gtbas3=0.\hf/gtmp2largexp= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , \PG=C01 [ X(N4)]\NImag=0\\ {N,N,N,P} \\0,1\N,0, , , \N, 0, , , \P,0, , , \N,0, , , \\Versi on=em64l-g09reva.02\state=1-a\mp2/gtbas1= \ccsd(t)/gtbas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \M P2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , \PG=C01 [X(N 3P1)]\NImag=0\\ {N,N,P,P} \\0,1\N,0, , , \N, 0, , , \P,0, , , \P,0, , , \\Versio S4
21 n=em64l-g09reva.02\state=1-a\mp2/gtbas1= \ccsd(t)/gtbas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP 2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , \PG=C01 [X(N 2P2)]\NImag=0\\ {N,P,P,P} \\0,1\P,0, , , \N,0, , , \P,0, , , \P,0, , , \\Version =EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \ MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , \PG=C01 [X(N1P3)]\NImag=0\\ {P,P,P,P} \\0,1\P,0, , , \P,0, , , \P,0, , , \P,0, , , \\ Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GT Bas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB 4= \G4MP2= \FreqCoord= , , , , , , , , , , , \PG=C01 [X(P4)]\NImag=0\\ {N,N,N,Si} \\0,1\N,0, , , \N,0, , , \Si,0, , , \N,0, , , \H, 0, , , \\Version=EM64L-G09RevA.02\ State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBa s2=0.\mp2/gtbas3=0.\hf/gtmp2largexp= \mp2/gtmp2largexp= \hf/gfhfb3= \hf/gfhfb4= \g4mp2= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(H1N3Si1)]\NImag=0\\ {N,N,Si,Si} \\0,1\N,0, , , \N,0, , , \Si,0, , , \Si,0, , , \H,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTM P2LargeXP= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , , , S5
22 , \PG=C01 [X(H2N2Si2)]\NImag=0\\ {N,Si,Si,Si} \\0,1\Si,0, , , \N,0, , , \Si,0, , , \Si,0, , , \H, 0, , , \H,0, , , \H,0, , , \\Version= EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/G TMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , , , , , , , \PG=C01 [X(H3N1Si3)]\NImag=0\\ {P,P,P,Si} \\0,1\P,0, , , \P, 0, , , \P,0, , , \Si,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\Stat e=1-a\mp2/gtbas1= \ccsd(t)/gtbas1= \mp2/gtbas2 =0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(H1P3Si1)]\NImag=0\\ {P,P,Si,Si} \\0,1\Si,0, , , \ P,0, , , \P,0, , , \Si,0, , , \H,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2La rgexp= \mp2/gtmp2largexp= \hf/gfhfb3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , , , , \PG=C01 [X(H2P2Si2)]\NImag=0\\ {N,N,P,Si} \\0,1\N,0, , , \N,0, , , \Si,0, , , \P,0, , , \H, 0, , , \\Version=EM64L-G09RevA.02\S tate=1-a\mp2/gtbas1= \ccsd(t)/gtbas1= \mp2/gtbas2 =0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(H1N2P1Si1)]\NImag=0\\ S6
23 {N,P,P,Si} \\0,1\P,0, , , \N,0, , , \Si,0, , , \P,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\Sta te=1-a\mp2/gtbas1= \ccsd(t)/gtbas1= \mp2/gtbas 2=0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(H1N1P2Si1)]\NImag=0\\ {N,P,Si,Si} \\0,1\P,0, , , \N,0, , , \Si,0, , , \Si,0, , , \H,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2 LargeXP= \MP2/GTMP2LargeXP= \HF/GFHFB3= \HF/GFHFB4= \G4MP2= \FreqCoord= , , , , , , , , , , , , , , , , , \PG=C01 [X(H2N1P1Si2)]\NImag=0\\ {Si,Si,Si,Si} \\0,1\Si,0, , , \Si,0, , , \Si,0, , , \Si,0, , , \H,0, , , \H,0, , , \H,0, , ,2.4502\H,0, ,2.3982, \\Version=EM64L-G09 RevA.02\State=1-A\MP2/GTBas1= \CCSD(T)/GTBas1= \MP2/GTBas2=0.\MP2/GTBas3=0.\HF/GTMP2LargeXP= \MP2/GTMP2L argexp= \hf/gfhfb3= \hf/gfhfb4= \g 4MP2= \FreqCoord= , , , , , , , , , , , , , , , , , , , , , , , \PG=C01 [X(H4Si4)]\NImag=0\\ S7
24 G4 archive entries for neutral singlet tetrahedranes {C,C,C,C} \\0,1\C,0, , , \C,0, , , \C,0, , , \C,0, , , \H,0, , , \H,0, , , \ H,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \MP4/GTBas1= \CCSD(T)/G3Bas1= \MP2/GTB as2= \mp4/gtbas2= \mp2/gtbas3= \mp4/gt Bas3= \HF/GTLargeXP= \MP2/GTLargeXP= \HF/GFHFB1= \HF/GFHFB2= \G4= \FreqCoo rd= , , , , , , , , , , , , , , , , , , , , , , , \PG=C01 [X(C4H4)]\ NImag=0\\ {C,C,C,N} \\0,1\N,0, , , \C,0, , , \C,0, , , \C,0, , , \H,0, , , \H,0, , , \H,0, , , \\Version=EM64L-G09RevA. 02\State=1-A\MP2/GTBas1= \MP4/GTBas1= \CCSD(T)/G3B as1= \mp2/gtbas2= \mp4/gtbas2= \mp2/g TBas3= \MP4/GTBas3= \HF/GTLargeXP= \MP 2/GTLargeXP= \HF/GFHFB1= \HF/GFHFB2= \G4= \FreqCoord= , , , , , , , , , , , , , , , , , , , , \PG=C01 [X(C3H3N1)]\NImag=0\\ {C,C,N,N} \\0,1\N,0, , , \N,0, , , \C,0, , , \C,0, , , \H,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \MP4/GTBa s1= \ccsd(t)/g3bas1= \mp2/gtbas2= \mp 4/GTBas2= \MP2/GTBas3= \MP4/GTBas3= \HF /GTLargeXP= \MP2/GTLargeXP= \HF/GFHFB1= \HF/GFHFB2= \G4= \FreqCoord= , , , , , , , , , , , , , , , , , \PG=C01 [X(C2H2N2)]\NImag=0\\ {C,N,N,N} \\0,1\N,0, , , \N,0, , , \N,0, , , \C,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/ GTBas1= \MP4/GTBas1= \CCSD(T)/G3Bas1= S8
25 6\MP2/GTBas2= \MP4/GTBas2= \MP2/GTBas3= \MP4/GTBas3= \HF/GTLargeXP= \MP2/GTLargeXP= \HF/GFHFB1= \HF/GFHFB2= \G4= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(C1H1N3)]\NImag=0\\ {C,C,C,P} \\0,1\C,0, , , \P,0, , , \C,0, , , \C,0, , , \H,0, , , \H,0, , , \ H,0, , , \\Version=EM64L-G09RevA.0 2\State=1-A\MP2/GTBas1= \MP4/GTBas1= \CCSD(T)/G3B as1= \mp2/gtbas2= \mp4/gtbas2= \mp2/gt Bas3= \MP4/GTBas3= \HF/GTLargeXP= \MP 2/GTLargeXP= \HF/GFHFB1= \HF/GFHFB2= \G4= \FreqCoord= , , , , , , , , , , , , , , , , , , , , \PG=C01 [X(C3H3P1)]\NImag=0\\ {C,C,P,P} \\0,1\P,0, , , \C,0, , , \P,0, , , \C,0, , , \H,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \MP4/G TBas1= \CCSD(T)/G3Bas1= \MP2/GTBas2= \MP4/GTBas2= \MP2/GTBas3= \MP4/GTBas3= \HF/GTLargeXP= \MP2/GTLargeXP= \HF/GFHFB1= \HF/GFHFB2= \G4= \FreqCoord= , , , , , , , , , , , , , , , , , \PG=C01 [X(C2H2P2)]\NImag=0\\ {C,P,P,P} \\0,1\P,0, , , \P,0, , , \P,0, , , \C,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/G TBas1= \MP4/GTBas1= \CCSD(T)/G3Bas1= \MP2/GTBas2= \MP4/GTBas2= \MP2/GTBas3= \MP4/GTBas3= \HF/GTLargeXP= \MP2/GTLarge XP= \HF/GFHFB1= \HF/GFHFB2= \G4= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(C1H1P3)]\NImag=0\\ {C,C,N,P} \\0,1\C,0, , , \C,0, , , \N,0, , , \P,0, , , \H,0, S9
26 801986, , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A\MP2/GTBas1= \MP 4/GTBas1= \CCSD(T)/G3Bas1= \MP2/GTBas2= \MP4/GTBas2= \MP2/GTBas3= \MP4/GTBas3= \HF/GTLargeXP= \MP2/GTLargeXP= \HF/GFHFB1= \HF/GFHFB2= \G4= \FreqCoord= , , , , , , , , , , , , , , , , , \PG=C01 [X(C2H2N1P1)]\NImag=0\\ {C,N,N,P} \\0,1\C,0, , , \N,0, , , \P,0, , , \N,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A \MP2/GTBas1= \MP4/GTBas1= \CCSD(T)/G3Bas1= \MP2/GTBas2= \MP4/GTBas2= \MP2/GTBas3= \MP4/GTBas3= \HF/GTLargeXP= \MP2/GTLargeX P= \HF/GFHFB1= \HF/GFHFB2= \G4= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(C1H1N2P1)]\NImag=0\\ {C,N,P,P} \\0,1\C,0, , , \N,0, , , \P,0, , , \P,0, , , \H,0, , , \\Version=EM64L-G09RevA.02\State=1-A \MP2/GTBas1= \MP4/GTBas1= \CCSD(T)/G3Bas1= \MP2/GTBas2= \MP4/GTBas2= \MP2/GTBas3= \MP4/GTBas3= \HF/GTLargeXP= \MP2/GTLarge XP= \HF/GFHFB1= \HF/GFHFB2= \G4= \FreqCoord= , , , , , , , , , , , , , , \PG=C01 [X(C1H1N1P2)]\NImag=0\\ {N,N,N,N} \\0,1\N,0, , , \N,0, , , \N,0, , , \N,0, , , \\Version=E M64L-G09RevA.02\State=1-A\MP2/GTBas1= \MP4/GTBas1= \CCSD(T)/G3Bas1= \MP2/GTBas2= \MP4/GTBas2= \MP2/GTBas3= \MP4/GTBas3= \HF/GTLargeXP = \MP2/GTLargeXP= \HF/GFHFB1= \HF/GFHF B2= \G4= \FreqCoord= , , , , , , , , , , , \PG=C 01 [X(N4)]\NImag=0\\ {N,N,N,P} \\0,1\N,0, , , \N,0, , , \P,0, , , \N,0, , , \\Version=EM64L- G09RevA.02\State=1-A\MP2/GTBas1= \MP4/GTBas1= \C CSD(T)/G3Bas1= \MP2/GTBas2= \MP4/GTBas2= S10
Table of Contents 1 Supplementary Data MCD
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
Supplementary Materials for. Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides
 Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Bifunctional Water Activation for Catalytic Hydration of Organonitriles
 Supporting Information (16 pages including the cover page) Bifunctional Water Activation for Catalytic Hydration of Organonitriles Prosenjit Daw, Arup Sinha, S. M. Wahidur Rahaman, Shrabani Dinda and Jitendra
Supporting Information (16 pages including the cover page) Bifunctional Water Activation for Catalytic Hydration of Organonitriles Prosenjit Daw, Arup Sinha, S. M. Wahidur Rahaman, Shrabani Dinda and Jitendra
Supporting Information. DFT Study of Pd(0)-Promoted Intermolecular C H Amination with. O-Benzoyl Hydroxylamines. List of Contents
 Supporting Information DFT Study of Pd(0)-Promoted Intermolecular C H Amination with O-Benzoyl Hydroxylamines Yunfei Zhou and Xiaoguang Bao* College of Chemistry, Chemical Engineering and Materials Science,
Supporting Information DFT Study of Pd(0)-Promoted Intermolecular C H Amination with O-Benzoyl Hydroxylamines Yunfei Zhou and Xiaoguang Bao* College of Chemistry, Chemical Engineering and Materials Science,
Supporting Information
 Supporting Information rigin of the Regio- and Stereoselectivity of Allylic Substitution of rganocopper Reagents Naohiko Yoshikai, Song-Lin Zhang, and Eiichi Nakamura* Department of Chemistry, The University
Supporting Information rigin of the Regio- and Stereoselectivity of Allylic Substitution of rganocopper Reagents Naohiko Yoshikai, Song-Lin Zhang, and Eiichi Nakamura* Department of Chemistry, The University
Supporting Information To. Microhydration of caesium compounds: Journal of Molecular Modeling
 Supporting Information To Microhydration of caesium compounds: Cs, CsOH, CsI and Cs 2 I 2 complexes with one to three H 2 O molecules of nuclear safety interest Journal of Molecular Modeling Mária Sudolská
Supporting Information To Microhydration of caesium compounds: Cs, CsOH, CsI and Cs 2 I 2 complexes with one to three H 2 O molecules of nuclear safety interest Journal of Molecular Modeling Mária Sudolská
Structural Expression of Exo-Anomeric Effect
 Supporting Information for Structural Expression of Exo-Anomeric Effect Elena R. Alonso, Isabel Peña, Carlos Cabezas, and José L. Alonso* Contents Table S1: Transition frequencies of conformer cc-β- 4
Supporting Information for Structural Expression of Exo-Anomeric Effect Elena R. Alonso, Isabel Peña, Carlos Cabezas, and José L. Alonso* Contents Table S1: Transition frequencies of conformer cc-β- 4
Carbohydrates in the gas phase: conformational preference of D-ribose and 2-deoxy-D-ribose
 New J. Chem ELECTRONIC SUPPLEMENTARY INFORMATION Carbohydrates in the gas phase: conformational preference of D-ribose and 2-deoxy-D-ribose Luis Miguel Azofra,*, María Mar Quesada-Moreno, Ibon Alkorta,
New J. Chem ELECTRONIC SUPPLEMENTARY INFORMATION Carbohydrates in the gas phase: conformational preference of D-ribose and 2-deoxy-D-ribose Luis Miguel Azofra,*, María Mar Quesada-Moreno, Ibon Alkorta,
Supporting Information for. Department of Chemistry, Vanderbilt University, Nashville, TN 37235
 Supporting Information for Functional Group Transformations in Derivatives of 1,4-Dihydrobenzo[1,2,4]triazinyl Radical Agnieszka Bodzioch, a, Minyan Zheng, a Piotr Kaszyński, a,b * Greta Utecht a a Organic
Supporting Information for Functional Group Transformations in Derivatives of 1,4-Dihydrobenzo[1,2,4]triazinyl Radical Agnieszka Bodzioch, a, Minyan Zheng, a Piotr Kaszyński, a,b * Greta Utecht a a Organic
An experimental and theoretical study of the gas phase kinetics of atomic chlorine reactions with CH 3 NH 2, (CH 3 ) 2 NH, and (CH 3 ) 3 N
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
ELECTRONIC SUPPORTING INFORMATION
 ELECTRONIC SUPPORTING INFORMATION Spectroscopic signatures of the carbon buckyonions C 60 @C 180 and C 60 @C 240 : a dispersion-corrected DFT study. Girolamo Casella, a Alessandro Bagno a and Giacomo Saielli*
ELECTRONIC SUPPORTING INFORMATION Spectroscopic signatures of the carbon buckyonions C 60 @C 180 and C 60 @C 240 : a dispersion-corrected DFT study. Girolamo Casella, a Alessandro Bagno a and Giacomo Saielli*
Mean bond enthalpy Standard enthalpy of formation Bond N H N N N N H O O O
 Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Electronic Supplementary Information DFT Characterization on the Mechanism of Water Splitting Catalyzed by Single-Ru-substituted Polyoxometalates
 Electronic Supplementary Information DFT Characterization on the Mechanism of Water Splitting Catalyzed by Single-Ru-substituted Polyoxometalates Zhong-Ling Lang, Guo-Chun Yang, Na-Na Ma, Shi-Zheng Wen,
Electronic Supplementary Information DFT Characterization on the Mechanism of Water Splitting Catalyzed by Single-Ru-substituted Polyoxometalates Zhong-Ling Lang, Guo-Chun Yang, Na-Na Ma, Shi-Zheng Wen,
Electronic structure and spectroscopy of HBr and HBr +
 Electronic structure and spectroscopy of HBr and HBr + Gabriel J. Vázquez 1 H. P. Liebermann 2 H. Lefebvre Brion 3 1 Universidad Nacional Autónoma de México Cuernavaca México 2 Universität Wuppertal Wuppertal
Electronic structure and spectroscopy of HBr and HBr + Gabriel J. Vázquez 1 H. P. Liebermann 2 H. Lefebvre Brion 3 1 Universidad Nacional Autónoma de México Cuernavaca México 2 Universität Wuppertal Wuppertal
Diels-Alder reaction of acenes with singlet and triplet oxygen - theoretical study of two-state reactivity
 Supporting Information Diels-Alder reaction of acenes with singlet and triplet oxygen - theoretical study of two-state reactivity A. Ravikumar Reddy and Michael Bendikov* Computational details: Density
Supporting Information Diels-Alder reaction of acenes with singlet and triplet oxygen - theoretical study of two-state reactivity A. Ravikumar Reddy and Michael Bendikov* Computational details: Density
Electronic Supplementary Information
 Electronic Supplementary Information The preferred all-gauche conformations in 3-fluoro-1,2-propanediol Laize A. F. Andrade, a Josué M. Silla, a Claudimar J. Duarte, b Roberto Rittner, b Matheus P. Freitas*,a
Electronic Supplementary Information The preferred all-gauche conformations in 3-fluoro-1,2-propanediol Laize A. F. Andrade, a Josué M. Silla, a Claudimar J. Duarte, b Roberto Rittner, b Matheus P. Freitas*,a
Fused Bis-Benzothiadiazoles as Electron Acceptors
 Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
1 P age. Hydrogen-abstraction reactions of methyl ethers, H 3 COCH 3-x (CH 3 ) x, x=0 2, by OH; Chong-Wen Zhou C 3
 Table S1. Rotational constants and vibrational frequencies of Reactants, Complexes, Transition States and Products Computed at the MP2/6-311G(d,p) level. Species I a, I b, I c (GHZ) Frequencies (cm -1
Table S1. Rotational constants and vibrational frequencies of Reactants, Complexes, Transition States and Products Computed at the MP2/6-311G(d,p) level. Species I a, I b, I c (GHZ) Frequencies (cm -1
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2006
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting information Chiral Thiourea-Based Bifunctional Organocatalysts in the Asymmetric Nitro- Michael Addition:
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting information Chiral Thiourea-Based Bifunctional Organocatalysts in the Asymmetric Nitro- Michael Addition:
Supplementary Information for
 Supplentary Information for Aggregation induced blue-shifted ission molecular picture from QM/MM study Qunyan Wu, a Tian Zhang, a Qian Peng,* b Dong Wang, a and Zhigang Shuai* a a Key Loratory of Organic
Supplentary Information for Aggregation induced blue-shifted ission molecular picture from QM/MM study Qunyan Wu, a Tian Zhang, a Qian Peng,* b Dong Wang, a and Zhigang Shuai* a a Key Loratory of Organic
Supporting Information
 SUPPORTING INFORMATION FOR: Trialkylstibine complexes of boron, aluminium, gallium and indium trihalides: synthesis, properties and bonding Victoria K. Greenacre, William Levason and Gillian Reid Chemistry,
SUPPORTING INFORMATION FOR: Trialkylstibine complexes of boron, aluminium, gallium and indium trihalides: synthesis, properties and bonding Victoria K. Greenacre, William Levason and Gillian Reid Chemistry,
Engineering Tunable Single and Dual Optical. Emission from Ru(II)-Polypyridyl Complexes. Through Excited State Design
 Engineering Tunable Single and Dual Optical Emission from Ru(II)-Polypyridyl Complexes Through Excited State Design Supplementary Information Julia Romanova 1, Yousif Sadik 1, M. R. Ranga Prabhath 1,,
Engineering Tunable Single and Dual Optical Emission from Ru(II)-Polypyridyl Complexes Through Excited State Design Supplementary Information Julia Romanova 1, Yousif Sadik 1, M. R. Ranga Prabhath 1,,
Supporting Information. A Combined Crossed Molecular Beams and ab Initio Investigation on the Formation of Vinylsulfidoboron (C 2 H
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information for A Combined Crossed Molecular Beams and ab Initio Investigation
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information for A Combined Crossed Molecular Beams and ab Initio Investigation
LP N to BD* C-C = BD C-C to BD* O-H = LP* C to LP* B =5.
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2016 MS No.: CP-ART-03-2016-002134.R1 Optical Response and Gas Sequestration Properties
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2016 MS No.: CP-ART-03-2016-002134.R1 Optical Response and Gas Sequestration Properties
 Table 1: Bond lengths (in Å) of rare-gas dimers without counterpoise correction for BSSE. a Method He 2 Ne 2 Ar 2 Kr 2 HeNe HeAr HeKr NeAr NeKr ArKr MSE MUE Reference 2.97 b 3.09 b 3.76 b 4.01 b 3.03 c
Table 1: Bond lengths (in Å) of rare-gas dimers without counterpoise correction for BSSE. a Method He 2 Ne 2 Ar 2 Kr 2 HeNe HeAr HeKr NeAr NeKr ArKr MSE MUE Reference 2.97 b 3.09 b 3.76 b 4.01 b 3.03 c
Διπλωματική Εργασία. Μελέτη των μηχανικών ιδιοτήτων των stents που χρησιμοποιούνται στην Ιατρική. Αντωνίου Φάνης
 Διπλωματική Εργασία Μελέτη των μηχανικών ιδιοτήτων των stents που χρησιμοποιούνται στην Ιατρική Αντωνίου Φάνης Επιβλέπουσες: Θεοδώρα Παπαδοπούλου, Ομότιμη Καθηγήτρια ΕΜΠ Ζάννη-Βλαστού Ρόζα, Καθηγήτρια
Διπλωματική Εργασία Μελέτη των μηχανικών ιδιοτήτων των stents που χρησιμοποιούνται στην Ιατρική Αντωνίου Φάνης Επιβλέπουσες: Θεοδώρα Παπαδοπούλου, Ομότιμη Καθηγήτρια ΕΜΠ Ζάννη-Βλαστού Ρόζα, Καθηγήτρια
DuPont Suva 95 Refrigerant
 Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Supporting Information
 Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany An Unusual Supramolecular Building Block: the Mixed Group 15/16 Element Ligand Complex [(Cp*Mo) 2 (µ,η 3 - P 3 )(µ,η 2 -PS)] Laurence J. Gregoriades,
Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany An Unusual Supramolecular Building Block: the Mixed Group 15/16 Element Ligand Complex [(Cp*Mo) 2 (µ,η 3 - P 3 )(µ,η 2 -PS)] Laurence J. Gregoriades,
10-π-electron arenes à la carte: Structure. Sr, Ba; n = 6-8) complexes
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2016 Supporting information 10-π-electron arenes à la carte: Structure and Bonding of
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2016 Supporting information 10-π-electron arenes à la carte: Structure and Bonding of
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information to the paper Theoretical Insights into the Separation
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information to the paper Theoretical Insights into the Separation
Manuscript submitted to the Journal of the American Society for Mass Spectrometry, September 2011.
 The Early Life of a Peptide Cation-Radical. Ground and Excited-State Trajectories of Electron-Based Peptide Dissociations During the First 330 Femtoseconds Christopher L. Moss, Wenkel Liang, Xiaosong Li,*
The Early Life of a Peptide Cation-Radical. Ground and Excited-State Trajectories of Electron-Based Peptide Dissociations During the First 330 Femtoseconds Christopher L. Moss, Wenkel Liang, Xiaosong Li,*
Supporting Information
 Supporting Information Wiley-VC 2009 69451 Weinheim, Germany S1 Supporting Information for: The Lowest Singlet and Triplet States of the Oxyallyl Diradical Takatoshi Ichino, Stephanie M. Villano, Adam
Supporting Information Wiley-VC 2009 69451 Weinheim, Germany S1 Supporting Information for: The Lowest Singlet and Triplet States of the Oxyallyl Diradical Takatoshi Ichino, Stephanie M. Villano, Adam
Supporting Information. A single probe to sense Al(III) colorimetrically and. Cd(II) by turn-on fluorescence in physiological
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 Supporting Information A single probe to sense Al(III) colorimetrically and Cd(II) by
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 Supporting Information A single probe to sense Al(III) colorimetrically and Cd(II) by
January 22, University of Minnesota, Minneapolis, Minnesota , USA
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 S-1 ELECTRONIC SUPPLEMENTARY INFORMATION January 22, 2017 Reaction of SO 2 with
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 S-1 ELECTRONIC SUPPLEMENTARY INFORMATION January 22, 2017 Reaction of SO 2 with
DuPont Suva. DuPont. Thermodynamic Properties of. Refrigerant (R-410A) Technical Information. refrigerants T-410A ENG
 Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Accessory Publication
 Accessory Publication Pitfalls in the Photoelectron Spectroscopic Investigations of Benzyne. Photoelectron Spectrum of Cyclopentadienylideneketene. Anna Chrostowska, A,C Genevieve Pfister-Guillouzo, A
Accessory Publication Pitfalls in the Photoelectron Spectroscopic Investigations of Benzyne. Photoelectron Spectrum of Cyclopentadienylideneketene. Anna Chrostowska, A,C Genevieve Pfister-Guillouzo, A
Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]
![Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)] Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]](/thumbs/89/98928029.jpg) d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
Supporting Information for: electron ligands: Complex formation, oxidation and
 Supporting Information for: The diverse reactions of PhI(OTf) 2 with common 2- electron ligands: Complex formation, oxidation and oxidative coupling Thomas P. Pell, Shannon A. Couchman, Sara Ibrahim, David
Supporting Information for: The diverse reactions of PhI(OTf) 2 with common 2- electron ligands: Complex formation, oxidation and oxidative coupling Thomas P. Pell, Shannon A. Couchman, Sara Ibrahim, David
DFT Kinetic Study of the Pyrolysis Mechanism of Toluene Used for Carbon Matrix
 2001 59 1, 17 21 ACTA CHIMICA SINICA Vol 59, 2001 No 1, 17 21 a,d Ξ a b b a a ( a b 710069) c d ( c 100083) ( d 710072) UB3LYP/ 3-21G 3 5 298 1 223 K : 963 K, 0 = 402 27 kj/ mol ; 963 K 1 223 K, E 0 =
2001 59 1, 17 21 ACTA CHIMICA SINICA Vol 59, 2001 No 1, 17 21 a,d Ξ a b b a a ( a b 710069) c d ( c 100083) ( d 710072) UB3LYP/ 3-21G 3 5 298 1 223 K : 963 K, 0 = 402 27 kj/ mol ; 963 K 1 223 K, E 0 =
Supporting Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Crotonols A and B, Two Rare Tigliane Diterpenoid
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Crotonols A and B, Two Rare Tigliane Diterpenoid
Reaction of Lithium Diethylamide with an Alkyl Bromide and Alkyl Benzenesulfonate: Origins of Alkylation, Elimination, and Sulfonation.
 Reaction of Lithium Diethylamide with an Alkyl omide and Alkyl Benzenesulfonate: rigins of Alkylation, Elimination, and ulfonation. Lekha Gupta, Antonio Ramírez and David B. Collum* Contribution from the
Reaction of Lithium Diethylamide with an Alkyl omide and Alkyl Benzenesulfonate: rigins of Alkylation, Elimination, and ulfonation. Lekha Gupta, Antonio Ramírez and David B. Collum* Contribution from the
Electronic Supplementary Information:
 Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Electronic Supplementary Information
 Electronic Supplementary Information NbCl 3 -catalyzed [2+2+2] intermolecular cycloaddition of alkynes and alkenes to 1,3-cyclohexadiene derivatives Yasushi Obora,* Keisuke Takeshita and Yasutaka Ishii*
Electronic Supplementary Information NbCl 3 -catalyzed [2+2+2] intermolecular cycloaddition of alkynes and alkenes to 1,3-cyclohexadiene derivatives Yasushi Obora,* Keisuke Takeshita and Yasutaka Ishii*
DuPont Suva 95 Refrigerant
 Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Computational study of the structure, UV-vis absorption spectra and conductivity of biphenylene-based polymers and their boron nitride analogues
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Computational study of the structure, UV-vis absorption spectra and conductivity of biphenylene-based
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Computational study of the structure, UV-vis absorption spectra and conductivity of biphenylene-based
Studies on the Binding Mechanism of Several Antibiotics and Human Serum Albumin
 2005 63 Vol. 63, 2005 23, 2169 2173 ACTA CHIMICA SINICA No. 23, 2169 2173 a,b a a a *,a ( a 130012) ( b 133002), 26 K A 1.98 10 4, 1.01 10 3, 1.38 10 3, 5.97 10 4 7.15 10 4 L mol 1, n 1.16, 0.86, 1.19,
2005 63 Vol. 63, 2005 23, 2169 2173 ACTA CHIMICA SINICA No. 23, 2169 2173 a,b a a a *,a ( a 130012) ( b 133002), 26 K A 1.98 10 4, 1.01 10 3, 1.38 10 3, 5.97 10 4 7.15 10 4 L mol 1, n 1.16, 0.86, 1.19,
of the methanol-dimethylamine complex
 Electronic Supplementary Information for: Fundamental and overtone virational spectroscopy, enthalpy of hydrogen ond formation and equilirium constant determination of the methanol-dimethylamine complex
Electronic Supplementary Information for: Fundamental and overtone virational spectroscopy, enthalpy of hydrogen ond formation and equilirium constant determination of the methanol-dimethylamine complex
Butadiene as a Ligand in Open Sandwich Compounds
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Butadiene as a Ligand in Open Sandwich Compounds Qunchao Fan, a Jia Fu, a Huidong
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Butadiene as a Ligand in Open Sandwich Compounds Qunchao Fan, a Jia Fu, a Huidong
Congruence Classes of Invertible Matrices of Order 3 over F 2
 International Journal of Algebra, Vol. 8, 24, no. 5, 239-246 HIKARI Ltd, www.m-hikari.com http://dx.doi.org/.2988/ija.24.422 Congruence Classes of Invertible Matrices of Order 3 over F 2 Ligong An and
International Journal of Algebra, Vol. 8, 24, no. 5, 239-246 HIKARI Ltd, www.m-hikari.com http://dx.doi.org/.2988/ija.24.422 Congruence Classes of Invertible Matrices of Order 3 over F 2 Ligong An and
Hydrogen Sorption Efficiency of Titanium Decorated Calix[4]pyrroles
![Hydrogen Sorption Efficiency of Titanium Decorated Calix[4]pyrroles Hydrogen Sorption Efficiency of Titanium Decorated Calix[4]pyrroles](/thumbs/84/89186739.jpg) Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 Hydrogen Sorption Efficiency of Titanium Decorated Calix[4]pyrroles Sandeep Kumar,
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 Hydrogen Sorption Efficiency of Titanium Decorated Calix[4]pyrroles Sandeep Kumar,
Supporting Information for
 Supporting Information for Hydrogen-Bridged Digermyl and Germylsilyl Cations N. Kordts, C. Borner, R. Panisch, W. Saak, T. Müller* Contents. 1. Computational Details 2. IR Spectroscopic Results 3. NMR-Spectroscopic
Supporting Information for Hydrogen-Bridged Digermyl and Germylsilyl Cations N. Kordts, C. Borner, R. Panisch, W. Saak, T. Müller* Contents. 1. Computational Details 2. IR Spectroscopic Results 3. NMR-Spectroscopic
Striking Difference between Succinimidomethyl and Phthalimidomethyl Radicals in Conjugate Addition to Alkylidenemalonate Initiated by Dimethylzinc
 Striking Difference between Succinimidomethyl and Phthalimidomethyl Radicals in Conjugate Addition to Alkylidenemalonate Initiated by Dimethylzinc Ken-ichi Yamada*, Yusuke Matsumoto, Shintaro Fujii, Takehito
Striking Difference between Succinimidomethyl and Phthalimidomethyl Radicals in Conjugate Addition to Alkylidenemalonate Initiated by Dimethylzinc Ken-ichi Yamada*, Yusuke Matsumoto, Shintaro Fujii, Takehito
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Polymer hemistry This journal is The Royal Society of hemistry 2011 Phosphoric and Phosphoramidic Acids as Bifunctional atalysts for the Ring-pening Polymerization
Electronic Supplementary Material (ESI) for Polymer hemistry This journal is The Royal Society of hemistry 2011 Phosphoric and Phosphoramidic Acids as Bifunctional atalysts for the Ring-pening Polymerization
k A = [k, k]( )[a 1, a 2 ] = [ka 1,ka 2 ] 4For the division of two intervals of confidence in R +
[a 1, a 2 ] = [ka 1,ka 2 ] 4For the division of two intervals of confidence in R + k A = [k, k]( )[a 1, a 2 ] = [ka 1,ka 2 ] 4For the division of two intervals of confidence in R +](/thumbs/73/69566903.jpg) Chapter 3. Fuzzy Arithmetic 3- Fuzzy arithmetic: ~Addition(+) and subtraction (-): Let A = [a and B = [b, b in R If x [a and y [b, b than x+y [a +b +b Symbolically,we write A(+)B = [a (+)[b, b = [a +b
Chapter 3. Fuzzy Arithmetic 3- Fuzzy arithmetic: ~Addition(+) and subtraction (-): Let A = [a and B = [b, b in R If x [a and y [b, b than x+y [a +b +b Symbolically,we write A(+)B = [a (+)[b, b = [a +b
Reaction of a Platinum Electrode for the Measurement of Redox Potential of Paddy Soil
 J. Jpn. Soc. Soil Phys. No. +*0, p.- +*,**1 Eh * ** Reaction of a Platinum Electrode for the Measurement of Redox Potential of Paddy Soil Daisuke MURAKAMI* and Tatsuaki KASUBUCHI** * The United Graduate
J. Jpn. Soc. Soil Phys. No. +*0, p.- +*,**1 Eh * ** Reaction of a Platinum Electrode for the Measurement of Redox Potential of Paddy Soil Daisuke MURAKAMI* and Tatsuaki KASUBUCHI** * The United Graduate
SUPPLEMENTARY INFORMATION
 Capture of Elusive Hydroxymethylene and its Fast Disappearance through Tunnelling Peter R. Schreiner a, Hans Peter Reisenauer a, Frank Pickard b, Andrew C. Simmonett b, Wesley D. Allen b, Edit Mátyus c
Capture of Elusive Hydroxymethylene and its Fast Disappearance through Tunnelling Peter R. Schreiner a, Hans Peter Reisenauer a, Frank Pickard b, Andrew C. Simmonett b, Wesley D. Allen b, Edit Mátyus c
Ethyl Nitroacetate in Aza-Henry Addition on Trifluoromethyl Aldimines: A Solvent-Free Procedure To Obtain Chiral Trifluoromethyl α,β-diamino Esters
 Supporting Information Ethyl Nitroacetate in Aza-Henry Addition on Trifluoromethyl Aldimines: A Solvent-Free Procedure To Obtain Chiral Trifluoromethyl α,β-diamino Esters Luca Parise, Alessia Pelagalli,
Supporting Information Ethyl Nitroacetate in Aza-Henry Addition on Trifluoromethyl Aldimines: A Solvent-Free Procedure To Obtain Chiral Trifluoromethyl α,β-diamino Esters Luca Parise, Alessia Pelagalli,
Supporting Information
 Supporting Information Chloroyttrium 2-(1-(arylimino)alkyl)quinolin-8-olate Complexes: Synthesis, Characterization, and Catalysis of the Ring-Opening Polymerization (ROP) of ε- Caprolactone (ε-cl) Wenjuan
Supporting Information Chloroyttrium 2-(1-(arylimino)alkyl)quinolin-8-olate Complexes: Synthesis, Characterization, and Catalysis of the Ring-Opening Polymerization (ROP) of ε- Caprolactone (ε-cl) Wenjuan
Si + Al Mg Fe + Mn +Ni Ca rim Ca p.f.u
 .6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
.6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
ΠΣΤΥΙΑΚΗ ΔΡΓΑΙΑ. Μειέηε Υξόλνπ Απνζηείξσζεο Κνλζέξβαο κε Τπνινγηζηηθή Ρεπζηνδπλακηθή. Αζαλαζηάδνπ Βαξβάξα
 ΣΔΥΝΟΛΟΓΙΚΟ ΔΚΠΑΙΓΔΤΣΙΚΟ ΙΓΡΤΜΑ ΘΔΑΛΟΝΙΚΗ ΥΟΛΗ ΣΔΥΝΟΛΟΓΙΑ ΣΡΟΦΙΜΩΝ & ΓΙΑΣΡΟΦΗ ΣΜΗΜΑ ΣΔΥΝΟΛΟΓΙΑ ΣΡΟΦΙΜΩΝ ΠΣΤΥΙΑΚΗ ΔΡΓΑΙΑ Μειέηε Υξόλνπ Απνζηείξσζεο Κνλζέξβαο κε Τπνινγηζηηθή Ρεπζηνδπλακηθή Αζαλαζηάδνπ Βαξβάξα
ΣΔΥΝΟΛΟΓΙΚΟ ΔΚΠΑΙΓΔΤΣΙΚΟ ΙΓΡΤΜΑ ΘΔΑΛΟΝΙΚΗ ΥΟΛΗ ΣΔΥΝΟΛΟΓΙΑ ΣΡΟΦΙΜΩΝ & ΓΙΑΣΡΟΦΗ ΣΜΗΜΑ ΣΔΥΝΟΛΟΓΙΑ ΣΡΟΦΙΜΩΝ ΠΣΤΥΙΑΚΗ ΔΡΓΑΙΑ Μειέηε Υξόλνπ Απνζηείξσζεο Κνλζέξβαο κε Τπνινγηζηηθή Ρεπζηνδπλακηθή Αζαλαζηάδνπ Βαξβάξα
Supporting Information
 Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
Dipole-Guided Electron Capture Causes Abnormal Dissociations of Phosphorylated Pentapeptides
 Supplementary Material for Dipole-Guided Electron Capture Causes Abnormal Dissociations of Phosphorylated Pentapeptides Christopher L. Moss, a Thomas W. Chung, a Jean A. Wyer, b Steen Brøndsted Nielsen,
Supplementary Material for Dipole-Guided Electron Capture Causes Abnormal Dissociations of Phosphorylated Pentapeptides Christopher L. Moss, a Thomas W. Chung, a Jean A. Wyer, b Steen Brøndsted Nielsen,
On a four-dimensional hyperbolic manifold with finite volume
 BULETINUL ACADEMIEI DE ŞTIINŢE A REPUBLICII MOLDOVA. MATEMATICA Numbers 2(72) 3(73), 2013, Pages 80 89 ISSN 1024 7696 On a four-dimensional hyperbolic manifold with finite volume I.S.Gutsul Abstract. In
BULETINUL ACADEMIEI DE ŞTIINŢE A REPUBLICII MOLDOVA. MATEMATICA Numbers 2(72) 3(73), 2013, Pages 80 89 ISSN 1024 7696 On a four-dimensional hyperbolic manifold with finite volume I.S.Gutsul Abstract. In
Solutions to the Schrodinger equation atomic orbitals. Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz
 Solutions to the Schrodinger equation atomic orbitals Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz ybridization Valence Bond Approach to bonding sp 3 (Ψ 2 s + Ψ 2 px + Ψ 2 py + Ψ 2 pz) sp 2 (Ψ 2 s + Ψ 2 px + Ψ 2 py)
Solutions to the Schrodinger equation atomic orbitals Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz ybridization Valence Bond Approach to bonding sp 3 (Ψ 2 s + Ψ 2 px + Ψ 2 py + Ψ 2 pz) sp 2 (Ψ 2 s + Ψ 2 px + Ψ 2 py)
Homework 3 Solutions
 Homework 3 Solutions Igor Yanovsky (Math 151A TA) Problem 1: Compute the absolute error and relative error in approximations of p by p. (Use calculator!) a) p π, p 22/7; b) p π, p 3.141. Solution: For
Homework 3 Solutions Igor Yanovsky (Math 151A TA) Problem 1: Compute the absolute error and relative error in approximations of p by p. (Use calculator!) a) p π, p 22/7; b) p π, p 3.141. Solution: For
Supporting Information
 Supporting Information Tris(pyrazolyl)methanides of the Alkaline Earth Metals - Influence of the Substitution Pattern on Stability and Degradation Christoph Müller, Alexander Koch, Helmar Görls, Sven Krieck,
Supporting Information Tris(pyrazolyl)methanides of the Alkaline Earth Metals - Influence of the Substitution Pattern on Stability and Degradation Christoph Müller, Alexander Koch, Helmar Görls, Sven Krieck,
Free Radical Initiated Coupling Reaction of Alcohols and. Alkynes: not C-O but C-C Bond Formation. Context. General information 2. Typical procedure 2
 Free Radical Initiated Coupling Reaction of Alcohols and Alkynes: not C-O but C-C Bond Formation Zhongquan Liu,* Liang Sun, Jianguo Wang, Jie Han, Yankai Zhao, Bo Zhou Institute of Organic Chemistry, Gannan
Free Radical Initiated Coupling Reaction of Alcohols and Alkynes: not C-O but C-C Bond Formation Zhongquan Liu,* Liang Sun, Jianguo Wang, Jie Han, Yankai Zhao, Bo Zhou Institute of Organic Chemistry, Gannan
Decomposition of Condensed Phase Energetic Materials: Interplay between Uni- and Bimolecular Mechanisms Supporting Information
 Decomposition of Condensed Phase Energetic Materials: Interplay between Uni- and Bimolecular Mechanisms Supporting Information a David Furman *, a Ronnie Kosloff, a Faina Dubnikova, b Sergey V. Zybin,
Decomposition of Condensed Phase Energetic Materials: Interplay between Uni- and Bimolecular Mechanisms Supporting Information a David Furman *, a Ronnie Kosloff, a Faina Dubnikova, b Sergey V. Zybin,
Enhancing σ/π-type Copper(I) thiophene Interactions by Metal Doping (Metal = Li, Na, K, Ca, Sc)
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Material (ESI) for Dalton Transactions This journal is The
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Material (ESI) for Dalton Transactions This journal is The
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) CPh 3 as a functional group in P-heterocyclic
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) CPh 3 as a functional group in P-heterocyclic
stability and aromaticity in the benzonitrile H 2 O complex with Na+ or Cl
 Electronic Supplementary Data A theoretical investigation on the cooperativity effect, reduced density gradient, stability and aromaticity in the benzonitrile H 2 O complex with Na+ or Cl Jiang-Bo Xie
Electronic Supplementary Data A theoretical investigation on the cooperativity effect, reduced density gradient, stability and aromaticity in the benzonitrile H 2 O complex with Na+ or Cl Jiang-Bo Xie
Resurvey of Possible Seismic Fissures in the Old-Edo River in Tokyo
 Bull. Earthq. Res. Inst. Univ. Tokyo Vol. 2.,**3 pp.,,3,.* * +, -. +, -. Resurvey of Possible Seismic Fissures in the Old-Edo River in Tokyo Kunihiko Shimazaki *, Tsuyoshi Haraguchi, Takeo Ishibe +, -.
Bull. Earthq. Res. Inst. Univ. Tokyo Vol. 2.,**3 pp.,,3,.* * +, -. +, -. Resurvey of Possible Seismic Fissures in the Old-Edo River in Tokyo Kunihiko Shimazaki *, Tsuyoshi Haraguchi, Takeo Ishibe +, -.
EE512: Error Control Coding
 EE512: Error Control Coding Solution for Assignment on Finite Fields February 16, 2007 1. (a) Addition and Multiplication tables for GF (5) and GF (7) are shown in Tables 1 and 2. + 0 1 2 3 4 0 0 1 2 3
EE512: Error Control Coding Solution for Assignment on Finite Fields February 16, 2007 1. (a) Addition and Multiplication tables for GF (5) and GF (7) are shown in Tables 1 and 2. + 0 1 2 3 4 0 0 1 2 3
상대론적고에너지중이온충돌에서 제트입자와관련된제동복사 박가영 인하대학교 윤진희교수님, 권민정교수님
 상대론적고에너지중이온충돌에서 제트입자와관련된제동복사 박가영 인하대학교 윤진희교수님, 권민정교수님 Motivation Bremsstrahlung is a major rocess losing energies while jet articles get through the medium. BUT it should be quite different from low energy
상대론적고에너지중이온충돌에서 제트입자와관련된제동복사 박가영 인하대학교 윤진희교수님, 권민정교수님 Motivation Bremsstrahlung is a major rocess losing energies while jet articles get through the medium. BUT it should be quite different from low energy
SUPPLEMENTAL INFORMATION. Fully Automated Total Metals and Chromium Speciation Single Platform Introduction System for ICP-MS
 Electronic Supplementary Material (ESI) for Journal of Analytical Atomic Spectrometry. This journal is The Royal Society of Chemistry 2018 SUPPLEMENTAL INFORMATION Fully Automated Total Metals and Chromium
Electronic Supplementary Material (ESI) for Journal of Analytical Atomic Spectrometry. This journal is The Royal Society of Chemistry 2018 SUPPLEMENTAL INFORMATION Fully Automated Total Metals and Chromium
* To whom correspondence should be addressed.
 SUPPORTING INFORMATION Computational design of a pincer phosphinito vanadium ((OPO)V) propane monoxygenation homogeneous catalyst based on the reduction-coupled oxo activation (ROA) mechanism Ross Fu,
SUPPORTING INFORMATION Computational design of a pincer phosphinito vanadium ((OPO)V) propane monoxygenation homogeneous catalyst based on the reduction-coupled oxo activation (ROA) mechanism Ross Fu,
The Role of Imidoselenium(II) Chlorides in the Formation of Cyclic Selenium
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 The Role of Imidoselenium(II) Chlorides in the Formation of Cyclic Selenium Imides via
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 The Role of Imidoselenium(II) Chlorides in the Formation of Cyclic Selenium Imides via
Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes
 Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes Nathalia B. D. Lima [a], José Diogo L. Dutra [a,b], Simone M. C. Gonçalves [a], Ricardo O.
Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes Nathalia B. D. Lima [a], José Diogo L. Dutra [a,b], Simone M. C. Gonçalves [a], Ricardo O.
2 Composition. Invertible Mappings
 Arkansas Tech University MATH 4033: Elementary Modern Algebra Dr. Marcel B. Finan Composition. Invertible Mappings In this section we discuss two procedures for creating new mappings from old ones, namely,
Arkansas Tech University MATH 4033: Elementary Modern Algebra Dr. Marcel B. Finan Composition. Invertible Mappings In this section we discuss two procedures for creating new mappings from old ones, namely,
Σπανό Ιωάννη Α.Μ. 148
 ΠΑΝΕΠΙΣΤΗΜΙΟ ΠΑΤΡΩΝ ΔΙΑΤΜΗΜΑΤΙΚΟ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΣΤΙΣ ΠΕΡΙΒΑΛΛΟΝΤΙΚΕΣ ΕΠΙΣΤΗΜΕΣ Ηλεκτροχημική εναπόθεση και μελέτη των ιδιοτήτων, λεπτών υμενίων μεταβατικών μετάλλων, για παραγωγή H2 Διπλωματική
ΠΑΝΕΠΙΣΤΗΜΙΟ ΠΑΤΡΩΝ ΔΙΑΤΜΗΜΑΤΙΚΟ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ ΣΤΙΣ ΠΕΡΙΒΑΛΛΟΝΤΙΚΕΣ ΕΠΙΣΤΗΜΕΣ Ηλεκτροχημική εναπόθεση και μελέτη των ιδιοτήτων, λεπτών υμενίων μεταβατικών μετάλλων, για παραγωγή H2 Διπλωματική
Supplementary!Information!
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015! Synthesis)and)characterisation)of)an)open1cage) fullerene)encapsulating)hydrogen)fluoride! Supplementary!Information!
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015! Synthesis)and)characterisation)of)an)open1cage) fullerene)encapsulating)hydrogen)fluoride! Supplementary!Information!
CHAPTER 48 APPLICATIONS OF MATRICES AND DETERMINANTS
 CHAPTER 48 APPLICATIONS OF MATRICES AND DETERMINANTS EXERCISE 01 Page 545 1. Use matrices to solve: 3x + 4y x + 5y + 7 3x + 4y x + 5y 7 Hence, 3 4 x 0 5 y 7 The inverse of 3 4 5 is: 1 5 4 1 5 4 15 8 3
CHAPTER 48 APPLICATIONS OF MATRICES AND DETERMINANTS EXERCISE 01 Page 545 1. Use matrices to solve: 3x + 4y x + 5y + 7 3x + 4y x + 5y 7 Hence, 3 4 x 0 5 y 7 The inverse of 3 4 5 is: 1 5 4 1 5 4 15 8 3
VBA Microsoft Excel. J. Comput. Chem. Jpn., Vol. 5, No. 1, pp (2006)
 J. Comput. Chem. Jpn., Vol. 5, No. 1, pp. 29 38 (2006) Microsoft Excel, 184-8588 2-24-16 e-mail: yosimura@cc.tuat.ac.jp (Received: July 28, 2005; Accepted for publication: October 24, 2005; Published on
J. Comput. Chem. Jpn., Vol. 5, No. 1, pp. 29 38 (2006) Microsoft Excel, 184-8588 2-24-16 e-mail: yosimura@cc.tuat.ac.jp (Received: July 28, 2005; Accepted for publication: October 24, 2005; Published on
Electronic Supporting Information
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Electronic Supporting Information robing steric influences on electrophilic phosphonium
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2018 Electronic Supporting Information robing steric influences on electrophilic phosphonium
ΔΙΕΡΕΥΝΗΣΗ ΤΗΣ ΣΕΞΟΥΑΛΙΚΗΣ ΔΡΑΣΤΗΡΙΟΤΗΤΑΣ ΤΩΝ ΓΥΝΑΙΚΩΝ ΚΑΤΑ ΤΗ ΔΙΑΡΚΕΙΑ ΤΗΣ ΕΓΚΥΜΟΣΥΝΗΣ ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ
 ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ Πτυχιακή Εργασία ΔΙΕΡΕΥΝΗΣΗ ΤΗΣ ΣΕΞΟΥΑΛΙΚΗΣ ΔΡΑΣΤΗΡΙΟΤΗΤΑΣ ΤΩΝ ΓΥΝΑΙΚΩΝ ΚΑΤΑ ΤΗ ΔΙΑΡΚΕΙΑ ΤΗΣ ΕΓΚΥΜΟΣΥΝΗΣ ΑΝΔΡΕΟΥ ΣΤΕΦΑΝΙΑ Λεμεσός 2012 i ii ΤΕΧΝΟΛΟΓΙΚΟ
ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΕΠΙΣΤΗΜΩΝ ΥΓΕΙΑΣ Πτυχιακή Εργασία ΔΙΕΡΕΥΝΗΣΗ ΤΗΣ ΣΕΞΟΥΑΛΙΚΗΣ ΔΡΑΣΤΗΡΙΟΤΗΤΑΣ ΤΩΝ ΓΥΝΑΙΚΩΝ ΚΑΤΑ ΤΗ ΔΙΑΡΚΕΙΑ ΤΗΣ ΕΓΚΥΜΟΣΥΝΗΣ ΑΝΔΡΕΟΥ ΣΤΕΦΑΝΙΑ Λεμεσός 2012 i ii ΤΕΧΝΟΛΟΓΙΚΟ
Supplementary Information
 9.4 6.60. 8.6 8.8 9.29 20.04 29.6 0.07 6. 40.92 4.64 06.0 6.29 6.0-0.00 Supplementary Information Conformational Analysis, Experimental and GIA-DFT C NMR Chemical Shift Calculation on 2 -Hydroxy-,4,-trimethoxy-chalcone
9.4 6.60. 8.6 8.8 9.29 20.04 29.6 0.07 6. 40.92 4.64 06.0 6.29 6.0-0.00 Supplementary Information Conformational Analysis, Experimental and GIA-DFT C NMR Chemical Shift Calculation on 2 -Hydroxy-,4,-trimethoxy-chalcone
CRASH COURSE IN PRECALCULUS
 CRASH COURSE IN PRECALCULUS Shiah-Sen Wang The graphs are prepared by Chien-Lun Lai Based on : Precalculus: Mathematics for Calculus by J. Stuwart, L. Redin & S. Watson, 6th edition, 01, Brooks/Cole Chapter
CRASH COURSE IN PRECALCULUS Shiah-Sen Wang The graphs are prepared by Chien-Lun Lai Based on : Precalculus: Mathematics for Calculus by J. Stuwart, L. Redin & S. Watson, 6th edition, 01, Brooks/Cole Chapter
Supporting Information. Crown Ether Complexes of Actinyls: A Computational Assessment of
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information Crown Ether Complexes of Actinyls: A Computational Assessment
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information Crown Ether Complexes of Actinyls: A Computational Assessment
6.1. Dirac Equation. Hamiltonian. Dirac Eq.
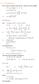 6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
Approximation of distance between locations on earth given by latitude and longitude
 Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
Matrices and Determinants
 Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
Supporting Information for Substituent Effects on the Properties of Borafluorenes
 Supporting Information for Substituent Effects on the Properties of Borafluorenes Mallory F. Smith, S. Joel Cassidy, Ian A. Adams, Monica Vasiliu, Deidra L. Gerlach, David Dixon*, Paul A. Rupar* Department
Supporting Information for Substituent Effects on the Properties of Borafluorenes Mallory F. Smith, S. Joel Cassidy, Ian A. Adams, Monica Vasiliu, Deidra L. Gerlach, David Dixon*, Paul A. Rupar* Department
 ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΤΜΗΜΑ ΦΥΣΙΚΗΣ ΙΑΤΜΗΜΑΤΙΚΟ ΜΕΤΑΠΤΥΧΙΑΚΟ ΠΡΟΓΡΑΜΜΑ ΣΠΟΥ ΩΝ ΝΑΝΟΕΠΙΣΤΗΜΕΣ & ΝΑΝΟΤΕΧΝΟΛΟΓΙΕΣ Ν&Ν ΓΡΑΦΕΝΙΟ : ΦΥΣΙΚΗ & ΕΦΑΡΜΟΓΕΣ ΛΑΜΠΡΙΝΟΣ Ι. ΜΙΧΑΗΛ ΕΠΙΒΛΕΠΩΝ ΚΑΘΗΓΗΤΗΣ:
ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΤΜΗΜΑ ΦΥΣΙΚΗΣ ΙΑΤΜΗΜΑΤΙΚΟ ΜΕΤΑΠΤΥΧΙΑΚΟ ΠΡΟΓΡΑΜΜΑ ΣΠΟΥ ΩΝ ΝΑΝΟΕΠΙΣΤΗΜΕΣ & ΝΑΝΟΤΕΧΝΟΛΟΓΙΕΣ Ν&Ν ΓΡΑΦΕΝΙΟ : ΦΥΣΙΚΗ & ΕΦΑΡΜΟΓΕΣ ΛΑΜΠΡΙΝΟΣ Ι. ΜΙΧΑΗΛ ΕΠΙΒΛΕΠΩΝ ΚΑΘΗΓΗΤΗΣ:
Optimizing Microwave-assisted Extraction Process for Paprika Red Pigments Using Response Surface Methodology
 2012 34 2 382-387 http / /xuebao. jxau. edu. cn Acta Agriculturae Universitatis Jiangxiensis E - mail ndxb7775@ sina. com 212018 105 W 42 2 min 0. 631 TS202. 3 A 1000-2286 2012 02-0382 - 06 Optimizing
2012 34 2 382-387 http / /xuebao. jxau. edu. cn Acta Agriculturae Universitatis Jiangxiensis E - mail ndxb7775@ sina. com 212018 105 W 42 2 min 0. 631 TS202. 3 A 1000-2286 2012 02-0382 - 06 Optimizing
ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΓΕΩΠΟΝΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΒΙΟΤΕΧΝΟΛΟΓΙΑΣ ΚΑΙ ΕΠΙΣΤΗΜΗΣ ΤΡΟΦΙΜΩΝ. Πτυχιακή εργασία
 ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΓΕΩΠΟΝΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΒΙΟΤΕΧΝΟΛΟΓΙΑΣ ΚΑΙ ΕΠΙΣΤΗΜΗΣ ΤΡΟΦΙΜΩΝ Πτυχιακή εργασία ΜΕΛΕΤΗ ΠΟΛΥΦΑΙΝΟΛΩΝ ΚΑΙ ΑΝΤΙΟΞΕΙΔΩΤΙΚΗΣ ΙΚΑΝΟΤΗΤΑΣ ΣΟΚΟΛΑΤΑΣ Αναστασία Σιάντωνα Λεμεσός
ΤΕΧΝΟΛΟΓΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΥΠΡΟΥ ΣΧΟΛΗ ΓΕΩΠΟΝΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΒΙΟΤΕΧΝΟΛΟΓΙΑΣ ΚΑΙ ΕΠΙΣΤΗΜΗΣ ΤΡΟΦΙΜΩΝ Πτυχιακή εργασία ΜΕΛΕΤΗ ΠΟΛΥΦΑΙΝΟΛΩΝ ΚΑΙ ΑΝΤΙΟΞΕΙΔΩΤΙΚΗΣ ΙΚΑΝΟΤΗΤΑΣ ΣΟΚΟΛΑΤΑΣ Αναστασία Σιάντωνα Λεμεσός
CHAPTER 25 SOLVING EQUATIONS BY ITERATIVE METHODS
 CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
C.S. 430 Assignment 6, Sample Solutions
 C.S. 430 Assignment 6, Sample Solutions Paul Liu November 15, 2007 Note that these are sample solutions only; in many cases there were many acceptable answers. 1 Reynolds Problem 10.1 1.1 Normal-order
C.S. 430 Assignment 6, Sample Solutions Paul Liu November 15, 2007 Note that these are sample solutions only; in many cases there were many acceptable answers. 1 Reynolds Problem 10.1 1.1 Normal-order
ΑΓΓΛΙΚΑ Ι. Ενότητα 7α: Impact of the Internet on Economic Education. Ζωή Κανταρίδου Τμήμα Εφαρμοσμένης Πληροφορικής
 Ενότητα 7α: Impact of the Internet on Economic Education Τμήμα Εφαρμοσμένης Πληροφορικής Άδειες Χρήσης Το παρόν εκπαιδευτικό υλικό υπόκειται σε άδειες χρήσης Creative Commons. Για εκπαιδευτικό υλικό, όπως
Ενότητα 7α: Impact of the Internet on Economic Education Τμήμα Εφαρμοσμένης Πληροφορικής Άδειες Χρήσης Το παρόν εκπαιδευτικό υλικό υπόκειται σε άδειες χρήσης Creative Commons. Για εκπαιδευτικό υλικό, όπως
ER-Tree (Extended R*-Tree)
 1-9825/22/13(4)768-6 22 Journal of Software Vol13, No4 1, 1, 2, 1 1, 1 (, 2327) 2 (, 3127) E-mail xhzhou@ustceducn,,,,,,, 1, TP311 A,,,, Elias s Rivest,Cleary Arya Mount [1] O(2 d ) Arya Mount [1] Friedman,Bentley
1-9825/22/13(4)768-6 22 Journal of Software Vol13, No4 1, 1, 2, 1 1, 1 (, 2327) 2 (, 3127) E-mail xhzhou@ustceducn,,,,,,, 1, TP311 A,,,, Elias s Rivest,Cleary Arya Mount [1] O(2 d ) Arya Mount [1] Friedman,Bentley
Divergent synthesis of various iminocyclitols from D-ribose
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 205 Divergent synthesis of various iminocyclitols from D-ribose Ramu Petakamsetty,
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 205 Divergent synthesis of various iminocyclitols from D-ribose Ramu Petakamsetty,
