1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions. 2.2 Gamma Transitions and Internal Conversion Coefficients
|
|
|
- Ζηναις Λιάπης
- 7 χρόνια πριν
- Προβολές:
Transcript
1 1 Decay Scheme Sn- decays by electron capture mainly to the In-m isomer which has a half-life of 99 minutes. After a sufficient time, the ratio of the In-m (99 min) and Sn- activities remains constant and is 1,0006. The values given here are the nuclides in equibium. Le Sn- se désintègre par capture électronique conduisant principalement à l isomère In-m ayant 99 min de période. Lorsque In-m et Sn- sont à l équilibre le rapport des activités de ces deux radionucléides est : 1,0006. Les valeurs sont données ici à l équilibre. 2 Nuclear Data T 1/2 ( Sn ) : 115,09 (3) d T 1/2 ( m In ) : 1,6579 (38) h Q ( Sn ) : 1036,0 (28) kev 2.1 Electron Capture Transitions Probability (%) Nature lg ft P K P L P M ɛ 0,3 6 (3) 0,00103 (4) Allowed 6,5 0,3 (3) 0,54 (20) ɛ 0,2 389 (3) 2,21 (8) 1st Forbidden 8,2 0,8490 (14) 0,121 (1) 0,0254 (5) ɛ 0,1 644 (3) 97,79 (8) 1st Forbidden 7,01 0,855 (1) 0,116 (1) 0,0241 (5) 2.2 Gamma Transitions and Internal Conversion Coefficients P γce α Multipolarity K α L α M α N α T (%) (10 2 ) (10 2 ) (10 3 ) γ 2,1(In) 255,134 (10) 2,21 (8) M133%E2 0,0396 (12) 0,549 (16) 0,1078 (32) 0,211 (6) 0,0464 (14) γ 3,2(In) 382,90 (8) 0, (3) γ 1,0(In) 391,698 (3) 100,00 (17) M4 0,437 (4) 8,58 (26) 1,70 (5) 3,77 (11) 0,540 (4) γ 3,1(In) 638,03 (8) 0,00097 (4) γ 2,0(In) 646,83 (1) 0, (2) [E3] INEEL / R.G. Helmer 1 14/07/ /10/2017
2 3 Atomic Data 3.1 In ω K : 0,851 (4) ω L : 0,0684 (20) n KL : 0,944 (4) X Radiations Relative probability X K Kα 2 24, ,3 Kα 1 24, Kβ 3 27,238 Kβ 1 27, ,8 Kβ 5 27,495 Kβ 2 27,861 Kβ 4 27,928 5,4 KO 2,3 27,939 L Ll 2,9 Lα 3,28-3,29 Lη 3,11 Lβ 3,49-3,79 Lγ 3,82-4, Auger Electrons Relative probability Auger K KLL 19,34-20, KLX 22,83-24,19 43,6 KXY 26,25-27,90 5,7 Auger L 2,0-4,2 INEEL / R.G. Helmer 2 14/07/ /10/2017
3 4 Electron Emissions Electrons (per 100 disint.) e AL (In) 2,0-4,2 116,3 (6) e AK (In) KLL 19,34-20,35 KLX 22,83-24,19 KXY 26,25-27,90 17,0 (5) ec 2,1 K (In) 227,194 (10) 0,0836 (41) ec 2,1 L (In) 250, ,404 0,0116 (6) ec 1,0 K (In) 363,758 (3) 28,39 (27) ec 1,0 L (In) 387, ,968 5,57 (17) ec 1,0 M (In) 390, ,255 1,104 (33) ec 1,0 N (In) 391, ,682 0,245 (7) ec 1,0 T (In) 363, ,682 35,31 (28) 5 Photon Emissions 5.1 X-Ray Emissions Photons (per 100 disint.) XL (In) 2,9-4,16 8,48 (19) } XKα 2 (In) 24, ,69 (21) Kα XKα 1 (In) 24,21 51,9 (3) XKβ 3 (In) 27,238 XKβ 1 (In) 27, ,58 (17) K β 1 XKβ 5 (In) 27,495 XKβ 2 (In) 27,861 XKβ 4 (In) 27,928 2,77 (10) K β 2 XKO 2,3 (In) 27, Gamma Emissions Photons (per 100 disint.) γ 2,1 (In) 255,134 (10) 2,11 (8) γ 3,2 (In) 382,90 (8) 0, (3) γ 1,0 (In) 391,698 (3) 64,97 (17) γ 3,1 (In) 638,03 (8) 0,00097 (4) γ 2,0 (In) 646,83 (1) 0, (2) INEEL / R.G. Helmer 3 14/07/ /10/2017
4 6 Main Production Modes Sn 112(n,γ)Sn m σ : 0,30 (4) barns Sn m(it)sn T 1/2 : 20 min { Sn 112(n,γ)Sn σ : 0,71 (10) barns Possible impurities: Sn 119m, Sn 121m, Sn 117m, Sn 123, Sn 125m 7 References - S.W.Barnes. Phys. Rev. 56 (1939) J.L.Lawson, J.M.Cork. Phys. Rev. 57 (1940) R.K.Girgis, R.Van Lieshout. Physica 24 (1958) S.B.Burson, H.A.Grench, L.C.Schmid. Phys. Rev. 115 (1959) R.C.Greenwood, E.Brannen. Phys. Rev. 122 (1961) 1849 (Gamma ray emission intensities) - R.C.Catura. Nucl. Instrum. Methods 32 (1965) H.E.Bosch, M.C.Simon, E.Szichman, L.Gatto, S.M.Abecasis. Phys. Rev. 159 (1967) H.Okamura, M.Ogawa, A.Mito. J. Inorg. Nucl. Chem. 29 (1967) B.Fogelberg, A.Bäcklin. Institute of Physics, University of Uppsala and Swedish Research Councils Laboratory Studsvik NP (1968) - E.Der Mateosian, M.Goldhaber. Phys. Rev. 186 (1969) H.van den Berg, M.McDonnell, M.K.Ramaswamy. Can. J. Phys. 47 (1969) E.Der Mateosian, M.Goldhaber. Phys. Rev. C2 (1970) I.W.Goodier, F.H.Hughes, M.J.Woods. Int. J. Appl. Radiat. Isotop. 21 (1970) J.Legrand, F.Lagoutine, J.P.Brethon. Int. J. Appl. Radiat. Isotop. 21 (1970) M.K.Ramaswamy. Phys. Rev. C1 (1970) R.J.Rousselin, Cl.Gauthier. Int. J. Appl. Radiat. Isotop. 21 (1970) J.M.Oottukulam, M.K.Ramaswamy. J. Am. Phys. 39 (1971) H.H.Hansen, E.De Roost, D.Mouchel, R.Vaninbroukx. Int. J. Appl. Radiat. Isotop. 22 (1971) 1 - F.Lagoutine, J.Legrand, C.Perrot, J.P.Brethon, J.Morel. Int. J. Appl. Radiat. Isotop. 23 (1972) J.F.Emery, S.A.Reynolds, E.I.Wyatt, G.I.Gleason. Nucl. Sci. Eng. 48 (1972) M.Ramaswamy. Conf. Vanderbilt Vol. 2 (1972) H.Inoue, Y.Yoshizawa, T.Morii. J. Phys. Soc. Jap. 34 (1973) S.Raman, N.B.Gove. Phys. Rev. C7 (1973) 1995 (lg ft) - H.J.Kim, R.L.Robinson. Phys. Rev. C9 (1974) 767 INEEL / R.G. Helmer 4 14/07/ /10/2017
5 - B.Bulow, M.Eriksson, G.G.Jonsson, Hagebo. Z Physik A275 (1975) V.Z.Kuttemperoor, R.A.Kobiske. Int. J. Appl. Radiat. Isotop. 26 (1975) A.A.Delucchi, R.A.Meyer. J. Inorg. Nucl. Chem. 38 (1976) 2135 (Gamma ray energies and emission probabilities) - W.Dietrich, A.Bäcklin. Z. Physik A276 (1976) F.Rösel, H.M.Fries, K.Alder, H.C.Pauli. At. Data. Nucl. Data Tables 21 (1978) 92 (Theoretical Internal Conversion Coefficients) - K.Heyde, M.Waroquier, R.A.Meyer. Phys. Rev. C17 (1978) 1219 (Gamma ray energies and emission probabilities) - H.Houtermans, O.Milosevic, F.Reichel. Int. J. Appl. Radiat. Isotop. 31 (1980) A.R.Rutledge, L.V.Smith, J.S.Merritt. NBS-SP-626 (1982) 5 - D.D.Hoppes, J.M.R.Hutchinson, F.J.Schima, M.P.Unterweger. NBS-SP-626 (1982) 85 - Y.Iwata, I.Yamamoto, Y.Yoshizawa. Int. J. Appl. Radiat. Isotop. 35 (1984) Zs.Nemeth, L.Lakosi, I.Pavlicsek, A.Veres. Int. J. Appl. Radiat. Isotop. 38 (1987) 63 - J.Blachot. Nucl. Data Sheets 59 (1990) 729 (Spin, multipolarities) - M.P.Unterweger, D.D.Hoppes, F.J.Schima. Nucl. Instrum. Methods A312 (1992) A.Mukherjee, S.Bhattacharya, B.Dasmahapatra. Appl. Rad. Isotopes 44 (1993) 731 (Gamma-ray emission intensities) - K.Debertin. Private Communication (1994) (Gamma-ray emission intensities) - G.Audi, A.H.Wapstra. Nucl. Phys. A595 (1995) 409 (Q) - X.Wen, K.Shizuma, S.Hamanaka, K.Iwatani, H.Hasai. Nucl. Instrum. Methods A397 (1997) R.G.Helmer, C.Van der Leun. Nucl. Instrum. Methods A450 (2000) 35 (Gamma ray energies) INEEL / R.G. Helmer 5 14/07/ /10/2017
6 LNE-LNHB/CEA - Table de Radionucléides Sn γ Emission intensities per 100 disintegrations 0 ε Sn /2 ; 0 115,09 (3) d 0,33 ns ,000 0,000 1/2,3/2 ; 1029,73 0, ,11 0, /2- ; 646,833 2,21 1,6579 h 1 6 4,97-1/2 ; 391,699 97,79 Stable 0 In Q = 1036 kev % ε = 100 9/2 ; 0 INEEL / R.G. Helmer Scheme page : 1/1 14/07/ /02/2004
1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions. 2.2 β Transitions
 1 Decay Scheme Le césium se désintègre essentiellement par émission bêta moins vers des niveaux excités du baryum. Cs- desintegrates mainly by beta minus emissions to excited levels in Ba-. 2 Nuclear Data
1 Decay Scheme Le césium se désintègre essentiellement par émission bêta moins vers des niveaux excités du baryum. Cs- desintegrates mainly by beta minus emissions to excited levels in Ba-. 2 Nuclear Data
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme Cf- disintegrates by alpha emissions mainly to the Cm-248 ground state level, and by spontaneous fission for 3,086(8) %. The average number of neutrons emitted by spontaneous fission is:
1 Decay Scheme Cf- disintegrates by alpha emissions mainly to the Cm-248 ground state level, and by spontaneous fission for 3,086(8) %. The average number of neutrons emitted by spontaneous fission is:
1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions. 2.2 Gamma Transitions and Internal Conversion Coefficients
 1 Decay Scheme Se disintegrates 100% by electron capture to excited levels and to the ground state of As. Le Se se désintègre à 100% par capture électronique vers des niveaux excités et le niveau fondamental
1 Decay Scheme Se disintegrates 100% by electron capture to excited levels and to the ground state of As. Le Se se désintègre à 100% par capture électronique vers des niveaux excités et le niveau fondamental
1 Decay Scheme. 2 Nuclear Data. 2.1 β Transitions
 1 Decay Scheme Rh- disintegrates by beta minus emission to the ground state and excited levels of Pd-. Le rhodium se désintègre par émission bêta principalement vers le niveau fondamental et les niveaux
1 Decay Scheme Rh- disintegrates by beta minus emission to the ground state and excited levels of Pd-. Le rhodium se désintègre par émission bêta principalement vers le niveau fondamental et les niveaux
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme Bi- disintegrates 97.91(3) % by beta minus emission to the levels in Po- and 2.09(3) % through alpha decay to Tl-209. Le bismuth se désintègre principalement par émissions bêta vers le polonium
1 Decay Scheme Bi- disintegrates 97.91(3) % by beta minus emission to the levels in Po- and 2.09(3) % through alpha decay to Tl-209. Le bismuth se désintègre principalement par émissions bêta vers le polonium
1 Decay Scheme. 2 Nuclear Data. 2.1 β Transitions
 1 Decay Scheme Np decays 87,8 (6) % by electron capture to U, 12,0 (6) % by beta minus emission to Pu, a possible alpha emission of 0,16 (6)% to Pa232 has not been observed. Le neptunium se désintègre
1 Decay Scheme Np decays 87,8 (6) % by electron capture to U, 12,0 (6) % by beta minus emission to Pu, a possible alpha emission of 0,16 (6)% to Pa232 has not been observed. Le neptunium se désintègre
1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions
 1 Decay Scheme I- disintegrates by electron capture mainly via the 159 level of Te- (97%). L iode se désintègre par capture électronique principalement vers le niveau excité de 159 du tellure, avec une
1 Decay Scheme I- disintegrates by electron capture mainly via the 159 level of Te- (97%). L iode se désintègre par capture électronique principalement vers le niveau excité de 159 du tellure, avec une
1 Decay Scheme. 2 Nuclear Data. 236m. 2.1 Electron Capture Transitions. 2.2 β Transitions
 1 Decay Scheme Np- (isomer state of Np-236, J=1, E1 =60 ) decays 53(1) % by electron capture to U-236 and 47(1) % by beta minus emission to Pu-236. Le neptunium 236 isomère se désintègre par capture électronique
1 Decay Scheme Np- (isomer state of Np-236, J=1, E1 =60 ) decays 53(1) % by electron capture to U-236 and 47(1) % by beta minus emission to Pu-236. Le neptunium 236 isomère se désintègre par capture électronique
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme Pa is a member of the natural U235 decay chain. Pa disintegrates by alpha emission to various excited levels and the ground state of Ac227. Le protactinium se désintègre par émissions alpha
1 Decay Scheme Pa is a member of the natural U235 decay chain. Pa disintegrates by alpha emission to various excited levels and the ground state of Ac227. Le protactinium se désintègre par émissions alpha
1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions. 2.2 Gamma Transitions and Internal Conversion Coefficients
 1 Decay Scheme Co decays 100% by electron capture to the excited levels of 706.42 kev (0.183%) and 136.47 kev (99.80%) in Fe. Le cobalt se désintègre par capture électronique vers des niveaux excités du
1 Decay Scheme Co decays 100% by electron capture to the excited levels of 706.42 kev (0.183%) and 136.47 kev (99.80%) in Fe. Le cobalt se désintègre par capture électronique vers des niveaux excités du
1 Decay Scheme. 2 Nuclear Data. 2.1 β Transitions. 2.2 Gamma Transitions and Internal Conversion Coefficients
 1 Decay Scheme Eu- disintegrates 100 % via beta minus disintegration to excited levels and to the ground state in Gd-. L europium se désintègre par émission bêta moins vers des niveaux excités et le niveau
1 Decay Scheme Eu- disintegrates 100 % via beta minus disintegration to excited levels and to the ground state in Gd-. L europium se désintègre par émission bêta moins vers des niveaux excités et le niveau
1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions. 2.2 Gamma Transitions and Internal Conversion Coefficients
 1 Decay Scheme In- disintegrates by > 99.99% electron capture via the excited level of 416.6 in Cd- and by < 0.01% electron capture via the isomer level in Cd- (T 1/2 = 48.5 min) at 396.2. Transitions
1 Decay Scheme In- disintegrates by > 99.99% electron capture via the excited level of 416.6 in Cd- and by < 0.01% electron capture via the isomer level in Cd- (T 1/2 = 48.5 min) at 396.2. Transitions
1 Decay Scheme. 2 Nuclear Data. 2.1 β + Transitions. 2.2 Electron Capture Transitions
 1 Decay Scheme Ni disintegrates by electron capture (56.6 %) and positron emission (43.4 %) to excited levels in Co. The transition to the ground state has not been observed. Le nickel se désintègre par
1 Decay Scheme Ni disintegrates by electron capture (56.6 %) and positron emission (43.4 %) to excited levels in Co. The transition to the ground state has not been observed. Le nickel se désintègre par
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme Bi- undergoes beta decay to Po- (64.07(7)%), and alpha decay to Tl-208 (35.93(7)%). 0.014(1)% of the beta branch undergoes prompt long-range alpha decay to Pb-208. Le bismuth se désintègre
1 Decay Scheme Bi- undergoes beta decay to Po- (64.07(7)%), and alpha decay to Tl-208 (35.93(7)%). 0.014(1)% of the beta branch undergoes prompt long-range alpha decay to Pb-208. Le bismuth se désintègre
1 Decay Scheme. 2 Nuclear Data. 2.1 β Transitions
 1 Decay Scheme Pb ground state (J π = 9/2 + ) decays by 100 % beta minus to Bi. The strongest branch (91.28 (12) %) decays directly to the ground state (J π = 9/2 ). Le plomb se désintègre par émissions
1 Decay Scheme Pb ground state (J π = 9/2 + ) decays by 100 % beta minus to Bi. The strongest branch (91.28 (12) %) decays directly to the ground state (J π = 9/2 ). Le plomb se désintègre par émissions
1 Decay Scheme. 2 Nuclear Data. 2.1 β Transitions. 2.2 Gamma Transitions and Internal Conversion Coefficients
 1 Decay Scheme Pb disintegrates by beta minus emission to excited levels and to the ground state level of Bi. Le plomb se désintègre par émission bêta moins vers des niveaux excités et le niveau fondamental
1 Decay Scheme Pb disintegrates by beta minus emission to excited levels and to the ground state level of Bi. Le plomb se désintègre par émission bêta moins vers des niveaux excités et le niveau fondamental
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme U- disintegrates by alpha emission mainly to the ground and 49- states in Th-232, and by 9,3 10 8 % spontaneous fission. L uranium se désintègre par émission alpha et par fission spontanée
1 Decay Scheme U- disintegrates by alpha emission mainly to the ground and 49- states in Th-232, and by 9,3 10 8 % spontaneous fission. L uranium se désintègre par émission alpha et par fission spontanée
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme Pu- decays approximately 100 % by beta minus emission to the ground state of Am- and 0,00244 % by alpha emission to levels of U-237. The spontaneous fission decay branching is less than
1 Decay Scheme Pu- decays approximately 100 % by beta minus emission to the ground state of Am- and 0,00244 % by alpha emission to levels of U-237. The spontaneous fission decay branching is less than
1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions. 2.2 Gamma Transitions and Internal Conversion Coefficients
 1 Decay Scheme Co disintegrates by 100% electron capture to the excited levels of 706.42 (0.18%), and 136.47 (99.82%) in Fe. Le cobalt se désintègre à 100 % par capture électronique principalement vers
1 Decay Scheme Co disintegrates by 100% electron capture to the excited levels of 706.42 (0.18%), and 136.47 (99.82%) in Fe. Le cobalt se désintègre à 100 % par capture électronique principalement vers
1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions. 2.2 Gamma Transitions and Internal Conversion Coefficients
 1 Decay Scheme Pb- disintegrates by electron capture to Tl- via excited levels. Le plomb se désintègre par capture électronique vers des niveaux excités du thallium. 2 Nuclear Data T 1/2 ( Pb ) : 51,929
1 Decay Scheme Pb- disintegrates by electron capture to Tl- via excited levels. Le plomb se désintègre par capture électronique vers des niveaux excités du thallium. 2 Nuclear Data T 1/2 ( Pb ) : 51,929
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme Am decays by emission of alpha particles to Np239, with a minute branch of 3.8 (7) 10 9 % by spontaneous fission. L américium se désintègre par émission alpha vers le neptunium 239. Un faible
1 Decay Scheme Am decays by emission of alpha particles to Np239, with a minute branch of 3.8 (7) 10 9 % by spontaneous fission. L américium se désintègre par émission alpha vers le neptunium 239. Un faible
1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions
 1 Decay Scheme - disintegrates 19.58 (11) % by beta plus emission and 8.2 (11) % by electron capture to Fe-. - emits gamma rays with energies up to 612 and the energies and emission probabilities for many
1 Decay Scheme - disintegrates 19.58 (11) % by beta plus emission and 8.2 (11) % by electron capture to Fe-. - emits gamma rays with energies up to 612 and the energies and emission probabilities for many
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme Uranium decays primarily by alpha decay to excited states in Th-228. A small branching of exotic decay via Ne-24 emission and a smaller branching of spontaneous fission have been reported.
1 Decay Scheme Uranium decays primarily by alpha decay to excited states in Th-228. A small branching of exotic decay via Ne-24 emission and a smaller branching of spontaneous fission have been reported.
1 Decay Scheme. 2 Nuclear Data. 2.1 β Transitions
 1 Decay Scheme Nd- disintegrates by beta minus emission to excited levels of Pm-. If a transition to the ground state level exists, it is less than 0.15 %. Le néodyme se désintègre par émission bêta moins
1 Decay Scheme Nd- disintegrates by beta minus emission to excited levels of Pm-. If a transition to the ground state level exists, it is less than 0.15 %. Le néodyme se désintègre par émission bêta moins
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme Cm decays 1% by alpha transitions to Pu38 and by spontaneous fission with branching fraction of 6.36 (14) E6 %. Le curium se désintègre à 1% par transition alpha vers le plutonium 38 et
1 Decay Scheme Cm decays 1% by alpha transitions to Pu38 and by spontaneous fission with branching fraction of 6.36 (14) E6 %. Le curium se désintègre à 1% par transition alpha vers le plutonium 38 et
1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions. 2.2 β + Transitions
 1 Decay Scheme Zr- (half-life of 78.42 h) undergoes 100% EC/positron decay (Q EC of 2832.8(28) kev) to various nuclear levels, including the metastable and ground states of Y-. Le zirconium se désintègre
1 Decay Scheme Zr- (half-life of 78.42 h) undergoes 100% EC/positron decay (Q EC of 2832.8(28) kev) to various nuclear levels, including the metastable and ground states of Y-. Le zirconium se désintègre
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme Cm- decays 100% by alpha transitions to Pu-240 and by spontaneous fission with branching fraction of 1.36 (1) E-4 %. Le curium se désintègre par émission alpha et par fission spontanée dans
1 Decay Scheme Cm- decays 100% by alpha transitions to Pu-240 and by spontaneous fission with branching fraction of 1.36 (1) E-4 %. Le curium se désintègre par émission alpha et par fission spontanée dans
1 Decay Scheme. 2 Nuclear Data. 2.1 α Transitions
 1 Decay Scheme Pu- decays 100% by alpha transitions to U-234. Most of the alpha decay populates the U-234 ground state (71.04 %) and the U-234 first excited level with energy of 43.50 (28.85 %). Branching
1 Decay Scheme Pu- decays 100% by alpha transitions to U-234. Most of the alpha decay populates the U-234 ground state (71.04 %) and the U-234 first excited level with energy of 43.50 (28.85 %). Branching
1 Decay Scheme. 2 Nuclear Data. 2.1 β Transitions
 1 Decay Scheme L iode se désintègre par émission bêta moins vers les niveaux excités de xénon, incluant l isomère xénon m de 11,962 (20) jours de période. L état d équilibre idéal, c est à dire l activité
1 Decay Scheme L iode se désintègre par émission bêta moins vers les niveaux excités de xénon, incluant l isomère xénon m de 11,962 (20) jours de période. L état d équilibre idéal, c est à dire l activité
1 Decay Scheme. 2 Nuclear Data. 2.1 Electron Capture Transitions
 1 Decay Scheme Ba disintegrates by electron capture mainly to two Cs excited levels of 437 kev (85.4%) and of 383 kev (14.5%) with three very minor branches to the 160 kev, 81 kev excited levels and the
1 Decay Scheme Ba disintegrates by electron capture mainly to two Cs excited levels of 437 kev (85.4%) and of 383 kev (14.5%) with three very minor branches to the 160 kev, 81 kev excited levels and the
Th, Ra, Rn, Po, Pb, Bi, & Tl K x-rays. Rn Kα1. Rn Kα2. 93( 227 Th)/Rn Kβ3. Ra Kα2. Po Kα2 /Bi K α1 79( 227 Th)/Po Kα1. Ra Kα1 /Bi K β1.
 Page -1-10 8 10 7 10 6 10 5 10 4 334 ( Th) Counts/Channel 10 3 10 2 10 1 49 ( Th)/ 50 ( Th)/ 50 ( Fr) 0 100 200 300 400 500 600 700 800 900 1000 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 Channel
Page -1-10 8 10 7 10 6 10 5 10 4 334 ( Th) Counts/Channel 10 3 10 2 10 1 49 ( Th)/ 50 ( Th)/ 50 ( Fr) 0 100 200 300 400 500 600 700 800 900 1000 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 2000 Channel
5.7. TABLE OF PHYSICAL CHARACTERISTICS OF RADIONUCLIDES MENTIONED IN THE EUROPEAN PHARMACOPOEIA
 01/2008:50700 5.7. TABLE OF PHYSICAL CHARACTERISTICS OF RADIONUCLIDES MENTIONED IN THE EUROPEAN PHARMACOPOEIA Thefollowingtableisgiventocompletethegeneral monograph Radiopharmaceutical preparations (0125).
01/2008:50700 5.7. TABLE OF PHYSICAL CHARACTERISTICS OF RADIONUCLIDES MENTIONED IN THE EUROPEAN PHARMACOPOEIA Thefollowingtableisgiventocompletethegeneral monograph Radiopharmaceutical preparations (0125).
RAPPORT CEA-R-6201 MARIE-MARTINE BÉ, CHRISTOPHE DULIEU, VANESSA CHISTÉ. "NUCLÉIDE-LARA - Bibliothèque des émissions alpha, X et gamma"
 RAPPORT CEA-R-6201 MARIE-MARTINE BÉ, CHRISTOPHE DULIEU, VANESSA CHISTÉ "NUCLÉIDE-LARA - Bibliothèque des émissions alpha, X et gamma" La bibliothèque NUCLÉIDE-LARA présente, pour près de 400 radionucléides
RAPPORT CEA-R-6201 MARIE-MARTINE BÉ, CHRISTOPHE DULIEU, VANESSA CHISTÉ "NUCLÉIDE-LARA - Bibliothèque des émissions alpha, X et gamma" La bibliothèque NUCLÉIDE-LARA présente, pour près de 400 radionucléides
Engineering Tunable Single and Dual Optical. Emission from Ru(II)-Polypyridyl Complexes. Through Excited State Design
 Engineering Tunable Single and Dual Optical Emission from Ru(II)-Polypyridyl Complexes Through Excited State Design Supplementary Information Julia Romanova 1, Yousif Sadik 1, M. R. Ranga Prabhath 1,,
Engineering Tunable Single and Dual Optical Emission from Ru(II)-Polypyridyl Complexes Through Excited State Design Supplementary Information Julia Romanova 1, Yousif Sadik 1, M. R. Ranga Prabhath 1,,
Butadiene as a Ligand in Open Sandwich Compounds
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Butadiene as a Ligand in Open Sandwich Compounds Qunchao Fan, a Jia Fu, a Huidong
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Butadiene as a Ligand in Open Sandwich Compounds Qunchao Fan, a Jia Fu, a Huidong
By R.L. Snyder (Revised March 24, 2005)
 Humidity Conversion By R.L. Snyder (Revised March 24, 2005) This Web page provides the equations used to make humidity conversions and tables o saturation vapor pressure. For a pd ile o this document,
Humidity Conversion By R.L. Snyder (Revised March 24, 2005) This Web page provides the equations used to make humidity conversions and tables o saturation vapor pressure. For a pd ile o this document,
MSWD = 1.06, probability = 0.39
 MSWD Institute of Geophysics and Planetary Physics Incremental Heating 36Ar(a) 37Ar(ca) 38Ar(cl) 39Ar(k) 40Ar(r) Age (Ma) 40Ar(r) (%) 1B15835D 670 C 4 0.000001 0.078288 0.000010 0.001470 0.010633 9.19
MSWD Institute of Geophysics and Planetary Physics Incremental Heating 36Ar(a) 37Ar(ca) 38Ar(cl) 39Ar(k) 40Ar(r) Age (Ma) 40Ar(r) (%) 1B15835D 670 C 4 0.000001 0.078288 0.000010 0.001470 0.010633 9.19
S
 MSWD Institute of Geophysics and Planetary Physics Incremental Heating 36Ar(a) 37Ar(ca) 38Ar(cl) 39Ar(k) 40Ar(r) Age (Ma) 40Ar(r) (%) 1B16079D 600 C 4 0.000034 0.033821 0.000003 0.001780 0.001016 0.72
MSWD Institute of Geophysics and Planetary Physics Incremental Heating 36Ar(a) 37Ar(ca) 38Ar(cl) 39Ar(k) 40Ar(r) Age (Ma) 40Ar(r) (%) 1B16079D 600 C 4 0.000034 0.033821 0.000003 0.001780 0.001016 0.72
Nuclear Physics 5. Name: Date: 8 (1)
 Name: Date: Nuclear Physics 5. A sample of radioactive carbon-4 decays into a stable isotope of nitrogen. As the carbon-4 decays, the rate at which the amount of nitrogen is produced A. decreases linearly
Name: Date: Nuclear Physics 5. A sample of radioactive carbon-4 decays into a stable isotope of nitrogen. As the carbon-4 decays, the rate at which the amount of nitrogen is produced A. decreases linearly
Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes
 Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes Nathalia B. D. Lima [a], José Diogo L. Dutra [a,b], Simone M. C. Gonçalves [a], Ricardo O.
Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes Nathalia B. D. Lima [a], José Diogo L. Dutra [a,b], Simone M. C. Gonçalves [a], Ricardo O.
P Ë ³μ,.. μ μ³μ²μ,.. ŠμÎ μ,.. μ μ,.. Š μ. ˆ œ ˆ Š Œˆ ŠˆŒ ƒ Œ Ÿ ˆŸ Š ˆ ˆ -ˆ ˆŠ
 P9-2008-102.. Ë ³μ,.. μ μ³μ²μ,.. ŠμÎ μ,.. μ μ,.. Š μ ˆ œ ˆ Š Œˆ ŠˆŒ ƒ Œ Ÿ ˆŸ Š ˆ ˆ -ˆ ˆŠ Ë ³μ... P9-2008-102 ˆ μ²ó μ Ô± μ³ Î ± ³ μ³ ²Ö μ²êî Ö Êα μ μ - ÉμÎ ± μ²êî É ÒÌ Ê ±μ ÒÌ Êαμ 48 Ö ²Ö É Ö μ μ ±²ÕÎ
P9-2008-102.. Ë ³μ,.. μ μ³μ²μ,.. ŠμÎ μ,.. μ μ,.. Š μ ˆ œ ˆ Š Œˆ ŠˆŒ ƒ Œ Ÿ ˆŸ Š ˆ ˆ -ˆ ˆŠ Ë ³μ... P9-2008-102 ˆ μ²ó μ Ô± μ³ Î ± ³ μ³ ²Ö μ²êî Ö Êα μ μ - ÉμÎ ± μ²êî É ÒÌ Ê ±μ ÒÌ Êαμ 48 Ö ²Ö É Ö μ μ ±²ÕÎ
P ƒ. μ μ², Œ.. ˆ μ,.. μ ± Î Š Ÿ ˆ Œ ˆŸ ˆ Ÿ Š ˆ. ² μ Ê ² μ Ò É Ì ± Ô± ³ É.
 P13-2011-120. ƒ. μ μ², Œ.. ˆ μ,.. μ ± Î Š Ÿ ˆ Œ ˆŸ ˆ Ÿ Š ˆ ² μ Ê ² μ Ò É Ì ± Ô± ³ É E-mail: sobolev@nrmail.jinr.ru μ μ². ƒ., ˆ μ Œ.., μ ± Î.. P13-2011-120 É μ ± ²Ö ³ Ö μ² ÒÌ Î Ö ÒÌ ±Í Ò É Ö Ô± ³ É ²Ó Ö
P13-2011-120. ƒ. μ μ², Œ.. ˆ μ,.. μ ± Î Š Ÿ ˆ Œ ˆŸ ˆ Ÿ Š ˆ ² μ Ê ² μ Ò É Ì ± Ô± ³ É E-mail: sobolev@nrmail.jinr.ru μ μ². ƒ., ˆ μ Œ.., μ ± Î.. P13-2011-120 É μ ± ²Ö ³ Ö μ² ÒÌ Î Ö ÒÌ ±Í Ò É Ö Ô± ³ É ²Ó Ö
An experimental and theoretical study of the gas phase kinetics of atomic chlorine reactions with CH 3 NH 2, (CH 3 ) 2 NH, and (CH 3 ) 3 N
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
Calculating the propagation delay of coaxial cable
 Your source for quality GNSS Networking Solutions and Design Services! Page 1 of 5 Calculating the propagation delay of coaxial cable The delay of a cable or velocity factor is determined by the dielectric
Your source for quality GNSS Networking Solutions and Design Services! Page 1 of 5 Calculating the propagation delay of coaxial cable The delay of a cable or velocity factor is determined by the dielectric
HOMEWORK#1. t E(x) = 1 λ = (b) Find the median lifetime of a randomly selected light bulb. Answer:
 HOMEWORK# 52258 李亞晟 Eercise 2. The lifetime of light bulbs follows an eponential distribution with a hazard rate of. failures per hour of use (a) Find the mean lifetime of a randomly selected light bulb.
HOMEWORK# 52258 李亞晟 Eercise 2. The lifetime of light bulbs follows an eponential distribution with a hazard rate of. failures per hour of use (a) Find the mean lifetime of a randomly selected light bulb.
P ² Ì μ Š ˆ Œˆ Š Œ Œˆ. ² μ Ê ² Nuclear Instruments and Methods in Physics Research.
 P1-2017-59.. ² Ì μ ˆ Š ˆ ˆ ƒˆ ˆˆ γ-š ƒ Œˆ Š ˆ Œˆ Š Œ Œˆ ² μ Ê ² Nuclear Instruments and Methods in Physics Research. Section A E-mail: zalikhanov@jinr.ru ² Ì μ.. P1-2017-59 μ ÒÏ ÔËË ±É μ É É Í γ-± Éμ μ
P1-2017-59.. ² Ì μ ˆ Š ˆ ˆ ƒˆ ˆˆ γ-š ƒ Œˆ Š ˆ Œˆ Š Œ Œˆ ² μ Ê ² Nuclear Instruments and Methods in Physics Research. Section A E-mail: zalikhanov@jinr.ru ² Ì μ.. P1-2017-59 μ ÒÏ ÔËË ±É μ É É Í γ-± Éμ μ
P Œ ²μ, ƒ.. μ ±μ,. ˆ. ˆ μ, Œ.. ƒê Éμ,. ƒ. ²μ,.. ³ É. ˆŒ ˆ Š ƒ Œ ˆ Ÿ ˆŸ 238 Uˆ 237 U, Œ ƒ Ÿ Š ˆˆ 238 U(γ,n) 237 U.
 P6-2009-30.. Œ ²μ, ƒ.. μ ±μ,. ˆ. ˆ μ, Œ.. ƒê Éμ,. ƒ. ²μ,.. ³ É ˆŒ ˆ Š ƒ Œ ˆ Ÿ ˆŸ 238 Uˆ 237 U, Œ ƒ Ÿ Š ˆˆ 238 U(γ,n) 237 U ² μ Ê ² μì ³ Ö, μ, μ² Ö Œ ²μ... ³ μ É Ê±ÉÊ μ μ ³ É ² ²Ö ² Ö 238U 237 U, μ²êî ³μ
P6-2009-30.. Œ ²μ, ƒ.. μ ±μ,. ˆ. ˆ μ, Œ.. ƒê Éμ,. ƒ. ²μ,.. ³ É ˆŒ ˆ Š ƒ Œ ˆ Ÿ ˆŸ 238 Uˆ 237 U, Œ ƒ Ÿ Š ˆˆ 238 U(γ,n) 237 U ² μ Ê ² μì ³ Ö, μ, μ² Ö Œ ²μ... ³ μ É Ê±ÉÊ μ μ ³ É ² ²Ö ² Ö 238U 237 U, μ²êî ³μ
April MD simulation of interaction between radiation damages and microstructure: voids, helium bubbles and grain boundaries
 2137-32 Joint ICTP-IAEA Advanced Workshop on Multi-Scale Modelling for Characterization and Basic Understanding of Radiation Damage Mechanisms in Materials 12-23 April 2010 MD simulation of interaction
2137-32 Joint ICTP-IAEA Advanced Workshop on Multi-Scale Modelling for Characterization and Basic Understanding of Radiation Damage Mechanisms in Materials 12-23 April 2010 MD simulation of interaction
Laboratory Studies on the Irradiation of Solid Ethane Analog Ices and Implications to Titan s Chemistry
 Laboratory Studies on the Irradiation of Solid Ethane Analog Ices and Implications to Titan s Chemistry 5th Titan Workshop at Kauai, Hawaii April 11-14, 2011 Seol Kim Outer Solar System Model Ices with
Laboratory Studies on the Irradiation of Solid Ethane Analog Ices and Implications to Titan s Chemistry 5th Titan Workshop at Kauai, Hawaii April 11-14, 2011 Seol Kim Outer Solar System Model Ices with
A life-table metamodel to support management of data deficient species, exemplified in sturgeons and shads. Electronic Supplementary Material
 A life-table metamodel to support management of data deficient species, exemplified in sturgeons and shads Electronic Supplementary Material Ivan Jarić 1,2*, Jörn Gessner 1 and Mirjana Lenhardt 3 1 Leibniz-Institute
A life-table metamodel to support management of data deficient species, exemplified in sturgeons and shads Electronic Supplementary Material Ivan Jarić 1,2*, Jörn Gessner 1 and Mirjana Lenhardt 3 1 Leibniz-Institute
ΒΙΟΓΡΑΦΙΚΟ ΣΗΜΕΙΩΜΑ. Μιχελακάκης Ηλίας Michelakakis Elias Εμμανουήλ.
 ΒΙΟΓΡΑΦΙΚΟ ΣΗΜΕΙΩΜΑ ΟΝΟΜΑΤΕΠΩΝΥΜΟ ΠΑΤΡΩΝΥΜΟ ΤΙΤΛΟΣ Μιχελακάκης Ηλίας Michelakakis Elias Εμμανουήλ Διδάκτωρ Φυσικός Παν/μίου Σάσσεξ Αγγλίας DPhil Doctor of Philosophy Nuclear Physics The University of Sussex
ΒΙΟΓΡΑΦΙΚΟ ΣΗΜΕΙΩΜΑ ΟΝΟΜΑΤΕΠΩΝΥΜΟ ΠΑΤΡΩΝΥΜΟ ΤΙΤΛΟΣ Μιχελακάκης Ηλίας Michelakakis Elias Εμμανουήλ Διδάκτωρ Φυσικός Παν/μίου Σάσσεξ Αγγλίας DPhil Doctor of Philosophy Nuclear Physics The University of Sussex
Zebra reaction or the recipe for heterodimeric zinc complexes synthesis
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 05 Supporting Information Zebra reaction or the recipe for heterodimeric zinc complexes synthesis
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 05 Supporting Information Zebra reaction or the recipe for heterodimeric zinc complexes synthesis
Lukasz STAWARZ. on Behalf of the Fermi LAT Collaboration.
 e-mail: kataoka.jun@waseda.jp 169 8555 3 4 1 739 8526 1 3 1 e-mail: fukazawa@hep01.hep1.hiroshima.u.ac.jp Lukasz STAWARZ 252 5210 3 1 1 e-mail: stawarz@astro.isas.jaxa.jp e-mail: rsato@astro.isas.jaxa.jp
e-mail: kataoka.jun@waseda.jp 169 8555 3 4 1 739 8526 1 3 1 e-mail: fukazawa@hep01.hep1.hiroshima.u.ac.jp Lukasz STAWARZ 252 5210 3 1 1 e-mail: stawarz@astro.isas.jaxa.jp e-mail: rsato@astro.isas.jaxa.jp
Bookmark and Index Summary
 Table of Isotopes CD- ROM Edition (Version 1.0, March 1996) The CD-ROM edition of the Table of Isotopes is an Adobe TM ACROBAT document. On- line help for ACROBAT is provided. The CD-ROM may be navigated
Table of Isotopes CD- ROM Edition (Version 1.0, March 1996) The CD-ROM edition of the Table of Isotopes is an Adobe TM ACROBAT document. On- line help for ACROBAT is provided. The CD-ROM may be navigated
2.1
 181 8588 2 21 1 e-mail: sekig@th.nao.ac.jp 1. G ab kt ab, (1) k 8pGc 4, G c 2. 1 2.1 308 2009 5 3 1 2) ( ab ) (g ab ) (K ab ) 1 2.2 3 1 (g ab, K ab ) 1 t a S n a a b a 2.3 a b i (t a ) 2 1 2.4 1 g ab ab
181 8588 2 21 1 e-mail: sekig@th.nao.ac.jp 1. G ab kt ab, (1) k 8pGc 4, G c 2. 1 2.1 308 2009 5 3 1 2) ( ab ) (g ab ) (K ab ) 1 2.2 3 1 (g ab, K ab ) 1 t a S n a a b a 2.3 a b i (t a ) 2 1 2.4 1 g ab ab
Nuclear Data Sheets for A = 110 *
 Nuclear Data Sheets 89, 481 (2000) doi:10.1006/ndsh.2000.0004 Nuclear Data Sheets for A = 110 * D. DE FRENNE AND E. JACOBS Vakgroep Subatomaire E(n) Stralingsfysica Proeftuinstraat 86, β 9000 Gent, Belgium
Nuclear Data Sheets 89, 481 (2000) doi:10.1006/ndsh.2000.0004 Nuclear Data Sheets for A = 110 * D. DE FRENNE AND E. JACOBS Vakgroep Subatomaire E(n) Stralingsfysica Proeftuinstraat 86, β 9000 Gent, Belgium
ˆ ˆˆ Œˆ C Z =47 50 Œ Œ ˆ ˆ Œ ˆ 23 ŒÔ
 P15-2014-58.. Š ³Ö,.. ŠÔ μ²² 1,.. ± μ,.. ²Ó,. ƒ. ²μ, ƒ.. μ ±μ,.. ³ É, ƒ. Ÿ. É μ Ê ˆ ˆ ˆ ˆ Œ ˆ ˆˆ Œˆ C Z =47 50 Œ Œ ˆ ˆ Œ ˆ 23 ŒÔ ² μ Ê ² Ÿ Ö Ë ± E-mail: karamian@nrmail.jinr.ru 1 ˆ ² μ É ²Ó ± Ö ² μ Éμ
P15-2014-58.. Š ³Ö,.. ŠÔ μ²² 1,.. ± μ,.. ²Ó,. ƒ. ²μ, ƒ.. μ ±μ,.. ³ É, ƒ. Ÿ. É μ Ê ˆ ˆ ˆ ˆ Œ ˆ ˆˆ Œˆ C Z =47 50 Œ Œ ˆ ˆ Œ ˆ 23 ŒÔ ² μ Ê ² Ÿ Ö Ë ± E-mail: karamian@nrmail.jinr.ru 1 ˆ ² μ É ²Ó ± Ö ² μ Éμ
P μ,. Œμ α 1,. ²μ ± 1,.. ϱ Î, Ÿ. Ê Í± 2 Œˆ ˆ Œ Š Ÿ Š Ÿ ˆ ˆŒ ˆˆ. ² μ Ê ² μ Ò É Ì ± Ô± ³ É
 P13-2009-117.. μ,. Œμ α 1,. ²μ ± 1,.. ϱ Î, Ÿ. Ê Í± 2 Œˆ ˆ Œ Š Ÿ Š Ÿ ˆ ˆŒ ˆˆ ² μ Ê ² μ Ò É Ì ± Ô± ³ É 1ˆ É ÉÊÉ Éμ³ μ Ô, ±Ä Ï, μ²óï 2 Ì μ²μ Î ± Ê É É, Õ ², μ²óï μ... P13-2009-117 μ ³ μ ³μ² ±Ê²Ö ÒÌ Êαμ
P13-2009-117.. μ,. Œμ α 1,. ²μ ± 1,.. ϱ Î, Ÿ. Ê Í± 2 Œˆ ˆ Œ Š Ÿ Š Ÿ ˆ ˆŒ ˆˆ ² μ Ê ² μ Ò É Ì ± Ô± ³ É 1ˆ É ÉÊÉ Éμ³ μ Ô, ±Ä Ï, μ²óï 2 Ì μ²μ Î ± Ê É É, Õ ², μ²óï μ... P13-2009-117 μ ³ μ ³μ² ±Ê²Ö ÒÌ Êαμ
DuPont Suva 95 Refrigerant
 Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
«Βασικές Αρχές της SPECT και PET Απεικόνισης»
 Βασικές Αρχές Πυρηνικής Ιατρικής «Βασικές Αρχές της SPECT και PET Απεικόνισης» Ι. Τσούγκος Εργαστήριο Ιατρικής Φυσικής, Παν/μιο Θεσσαλίας ΙΣΤΟΡΙΚΗ ΑΝΑΔΡΟΜΗ 1896: Henry Becquerel και το ζεύγος Curie ήταν
Βασικές Αρχές Πυρηνικής Ιατρικής «Βασικές Αρχές της SPECT και PET Απεικόνισης» Ι. Τσούγκος Εργαστήριο Ιατρικής Φυσικής, Παν/μιο Θεσσαλίας ΙΣΤΟΡΙΚΗ ΑΝΑΔΡΟΜΗ 1896: Henry Becquerel και το ζεύγος Curie ήταν
ΘΕΩΡΗΤΙΚΗ ΚΑΙ ΠΕΙΡΑΜΑΤΙΚΗ ΙΕΡΕΥΝΗΣΗ ΤΗΣ ΙΕΡΓΑΣΙΑΣ ΣΚΛΗΡΥΝΣΗΣ ΙΑ ΛΕΙΑΝΣΕΩΣ
 ΠΑΝΕΠΙΣΤΗΜΙΟ ΠΑΤΡΩΝ ΠΟΛΥΤΕΧΝΙΚΗ ΣΧΟΛΗ ΤΜΗΜΑ ΜΗΧΑΝΟΛΟΓΩΝ ΚΑΙ ΑΕΡΟΝΑΥΠΗΓΩΝ ΜΗΧΑΝΙΚΩΝ ΕΡΓΑΣΤΗΡΙΟ ΣΥΣΤΗΜΑΤΩΝ ΠΑΡΑΓΩΓΗΣ ΚΑΙ ΑΥΤΟΜΑΤΙΣΜΟΥ / ΥΝΑΜΙΚΗΣ & ΘΕΩΡΙΑΣ ΜΗΧΑΝΩΝ ΙΕΥΘΥΝΤΗΣ: Καθηγητής Γ. ΧΡΥΣΟΛΟΥΡΗΣ Ι ΑΚΤΟΡΙΚΗ
ΠΑΝΕΠΙΣΤΗΜΙΟ ΠΑΤΡΩΝ ΠΟΛΥΤΕΧΝΙΚΗ ΣΧΟΛΗ ΤΜΗΜΑ ΜΗΧΑΝΟΛΟΓΩΝ ΚΑΙ ΑΕΡΟΝΑΥΠΗΓΩΝ ΜΗΧΑΝΙΚΩΝ ΕΡΓΑΣΤΗΡΙΟ ΣΥΣΤΗΜΑΤΩΝ ΠΑΡΑΓΩΓΗΣ ΚΑΙ ΑΥΤΟΜΑΤΙΣΜΟΥ / ΥΝΑΜΙΚΗΣ & ΘΕΩΡΙΑΣ ΜΗΧΑΝΩΝ ΙΕΥΘΥΝΤΗΣ: Καθηγητής Γ. ΧΡΥΣΟΛΟΥΡΗΣ Ι ΑΚΤΟΡΙΚΗ
Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative cleavage of cyclic ethers. Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative
DuPont Suva. DuPont. Thermodynamic Properties of. Refrigerant (R-410A) Technical Information. refrigerants T-410A ENG
 Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Š Ÿ Š Ÿ Ÿ ˆ Œ ˆŠ -280
 Ó³ Ÿ.. 2012.. 9, º 8.. 89Ä97 Š Ÿ Š Ÿ Ÿ ˆ Œ ˆŠ -280 ƒ. ƒ. ƒê²ó ±Ö,.. Ê, ƒ.. Š ³ÒÏ,.. Š ³ÒÏ,. ±μ Ñ Ò É ÉÊÉ Ö ÒÌ ² μ, Ê ³ É É Ö Ò μ±μî ÉμÉ Ö Ê ±μ ÖÕÐ Ö É ³ ÉÒ ³μ μ μ Éμ Ö - ÒÌ ±Í ³. ƒ.. ² μ Ñ μ μ É ÉÊÉ Ö
Ó³ Ÿ.. 2012.. 9, º 8.. 89Ä97 Š Ÿ Š Ÿ Ÿ ˆ Œ ˆŠ -280 ƒ. ƒ. ƒê²ó ±Ö,.. Ê, ƒ.. Š ³ÒÏ,.. Š ³ÒÏ,. ±μ Ñ Ò É ÉÊÉ Ö ÒÌ ² μ, Ê ³ É É Ö Ò μ±μî ÉμÉ Ö Ê ±μ ÖÕÐ Ö É ³ ÉÒ ³μ μ μ Éμ Ö - ÒÌ ±Í ³. ƒ.. ² μ Ñ μ μ É ÉÊÉ Ö
Ó³ Ÿ , º 7(163).. 793Ä797 ˆ ˆŠ ˆ ˆŠ Š ˆ. .. Ëμ μ. Î ± É ÉÊÉ ³..., Œμ ±
 Ó³ Ÿ. 2010.. 7, º 7(163).. 793Ä797 ˆ ˆŠ ˆ ˆŠ Š ˆ Š ˆ œ Š Œ ˆ Œ.. Ëμ μ Î ± É ÉÊÉ ³..., Œμ ± ² É Î ± ³μÉ μ Ëμ ³ μ ²Ó μéμî ÒÌ Ô² ±É μ ÒÌ Êαμ, Ö ±μéμ ÒÌ Î É Î μ É ² μ μ ³, Éμ± ³, ÒÏ ÕÐ ³ ²Ó μ Î Éμ± ²Ó. Ê
Ó³ Ÿ. 2010.. 7, º 7(163).. 793Ä797 ˆ ˆŠ ˆ ˆŠ Š ˆ Š ˆ œ Š Œ ˆ Œ.. Ëμ μ Î ± É ÉÊÉ ³..., Œμ ± ² É Î ± ³μÉ μ Ëμ ³ μ ²Ó μéμî ÒÌ Ô² ±É μ ÒÌ Êαμ, Ö ±μéμ ÒÌ Î É Î μ É ² μ μ ³, Éμ± ³, ÒÏ ÕÐ ³ ²Ó μ Î Éμ± ²Ó. Ê
Electronic Supplementary Information:
 Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
(1) Describe the process by which mercury atoms become excited in a fluorescent tube (3)
 Q1. (a) A fluorescent tube is filled with mercury vapour at low pressure. In order to emit electromagnetic radiation the mercury atoms must first be excited. (i) What is meant by an excited atom? (1) (ii)
Q1. (a) A fluorescent tube is filled with mercury vapour at low pressure. In order to emit electromagnetic radiation the mercury atoms must first be excited. (i) What is meant by an excited atom? (1) (ii)
P É Ô Ô² 1,2,.. Ò± 1,.. ±μ 1,. ƒ. ±μ μ 1,.Š. ±μ μ 1, ˆ.. Ê Ò 1,.. Ê Ò 1 Œˆ ˆŸ. ² μ Ê ² μ Ì μ ÉÓ. É μ ±, Ì μé μ Ò É μ Ò ² μ Ö
 P11-2015-60. É Ô Ô² 1,2,.. Ò± 1,.. ±μ 1,. ƒ. ±μ μ 1,.Š. ±μ μ 1, ˆ.. Ê Ò 1,.. Ê Ò 1 Œ Œ ˆ Š Œ ˆ ˆ Œˆ ˆŸ ƒ Š ˆŒ Š ² μ Ê ² μ Ì μ ÉÓ. É μ ±, Ì μé μ Ò É μ Ò ² μ Ö 1 Ñ Ò É ÉÊÉ Ö ÒÌ ² μ, Ê 2 Œμ μ²ó ± μ Ê É Ò
P11-2015-60. É Ô Ô² 1,2,.. Ò± 1,.. ±μ 1,. ƒ. ±μ μ 1,.Š. ±μ μ 1, ˆ.. Ê Ò 1,.. Ê Ò 1 Œ Œ ˆ Š Œ ˆ ˆ Œˆ ˆŸ ƒ Š ˆŒ Š ² μ Ê ² μ Ì μ ÉÓ. É μ ±, Ì μé μ Ò É μ Ò ² μ Ö 1 Ñ Ò É ÉÊÉ Ö ÒÌ ² μ, Ê 2 Œμ μ²ó ± μ Ê É Ò
NUCLEAR WALLET CARDS
 NUCLEAR WALLET CARDS (Fifth edition) JULY 1995 JAGDISH K. TULI NATIONAL NUCLEAR DATA CENTER for The U.S. Nuclear Data Network Supported by The Division of Nuclear Physics, Office of High Energy and Nuclear
NUCLEAR WALLET CARDS (Fifth edition) JULY 1995 JAGDISH K. TULI NATIONAL NUCLEAR DATA CENTER for The U.S. Nuclear Data Network Supported by The Division of Nuclear Physics, Office of High Energy and Nuclear
ˆŒ œ ƒ ƒ ˆ ˆŸ ˆ Š ˆ 137 Cs Š ˆ Œ.
 Ó³ Ÿ. 2017.. 14, º 6(211).. 630Ä636 ˆ ˆŠ Œ ˆ ˆ Œ ƒ Ÿ. Š ˆŒ ˆ Š ˆŸ ˆŸ ˆŒ œ ƒ ƒ ˆ ˆŸ ˆ Š ˆ 137 Cs Š ˆ Œ. œ.., 1,.. ³,. ƒ. Š ² ±μ,.. ³ ±,.. ³ μ,. ˆ. É ²μ,. ˆ. ÕÉÕ ±μ, ƒ.. Ë,, ˆ.. ±μ ˆ É ÉÊÉ μ Ð Ë ± ³.. Œ.
Ó³ Ÿ. 2017.. 14, º 6(211).. 630Ä636 ˆ ˆŠ Œ ˆ ˆ Œ ƒ Ÿ. Š ˆŒ ˆ Š ˆŸ ˆŸ ˆŒ œ ƒ ƒ ˆ ˆŸ ˆ Š ˆ 137 Cs Š ˆ Œ. œ.., 1,.. ³,. ƒ. Š ² ±μ,.. ³ ±,.. ³ μ,. ˆ. É ²μ,. ˆ. ÕÉÕ ±μ, ƒ.. Ë,, ˆ.. ±μ ˆ É ÉÊÉ μ Ð Ë ± ³.. Œ.
Electronic Supplementary Information DFT Characterization on the Mechanism of Water Splitting Catalyzed by Single-Ru-substituted Polyoxometalates
 Electronic Supplementary Information DFT Characterization on the Mechanism of Water Splitting Catalyzed by Single-Ru-substituted Polyoxometalates Zhong-Ling Lang, Guo-Chun Yang, Na-Na Ma, Shi-Zheng Wen,
Electronic Supplementary Information DFT Characterization on the Mechanism of Water Splitting Catalyzed by Single-Ru-substituted Polyoxometalates Zhong-Ling Lang, Guo-Chun Yang, Na-Na Ma, Shi-Zheng Wen,
Si + Al Mg Fe + Mn +Ni Ca rim Ca p.f.u
 .6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
.6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
CHAPTER 25 SOLVING EQUATIONS BY ITERATIVE METHODS
 CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
DuPont Suva 95 Refrigerant
 Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Dipole-Guided Electron Capture Causes Abnormal Dissociations of Phosphorylated Pentapeptides
 Supplementary Material for Dipole-Guided Electron Capture Causes Abnormal Dissociations of Phosphorylated Pentapeptides Christopher L. Moss, a Thomas W. Chung, a Jean A. Wyer, b Steen Brøndsted Nielsen,
Supplementary Material for Dipole-Guided Electron Capture Causes Abnormal Dissociations of Phosphorylated Pentapeptides Christopher L. Moss, a Thomas W. Chung, a Jean A. Wyer, b Steen Brøndsted Nielsen,
IB PHYSICS HL REVIEW PACKET: NUCLEAR PHYSICS
 NAME IB PHYSICS HL REVIEW PACKET: NUCLEAR PHYSICS 1. This question is about nuclear reactions. (a) Complete the table below, by placing a tick ( ) in the relevant columns, to show how an increase in each
NAME IB PHYSICS HL REVIEW PACKET: NUCLEAR PHYSICS 1. This question is about nuclear reactions. (a) Complete the table below, by placing a tick ( ) in the relevant columns, to show how an increase in each
Derivation of Optical-Bloch Equations
 Appendix C Derivation of Optical-Bloch Equations In this appendix the optical-bloch equations that give the populations and coherences for an idealized three-level Λ system, Fig. 3. on page 47, will be
Appendix C Derivation of Optical-Bloch Equations In this appendix the optical-bloch equations that give the populations and coherences for an idealized three-level Λ system, Fig. 3. on page 47, will be
10-π-electron arenes à la carte: Structure. Sr, Ba; n = 6-8) complexes
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2016 Supporting information 10-π-electron arenes à la carte: Structure and Bonding of
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2016 Supporting information 10-π-electron arenes à la carte: Structure and Bonding of
P ˆ.. ƒê ²μ 1,.. Œ ² ±μ 1,..Šμ Í,.. ʳ,.. μ μ 2. ˆ ˆŸ Š Š ˆ ƒ ˆŒ œ ƒ Œ ƒ ƒ Š-Š ˆ 10- Œ ˆ. ( ), Œμ ± Œμ ± 1 μ Ò É Ì μ²μ ±μ³ μ ÉÒ ±Êʳ ÒÌ μ μ
 P9-2017-78 ˆ.. ƒê ²μ 1,.. Œ ² ±μ 1,..Šμ Í,.. ʳ,.. μ μ 2 ˆ ˆŸ Š Š ˆ ƒ ˆŒ œ ƒ Œ ƒ ƒ Š-Š ˆ 10- Œ ˆ 1 μ Ò É Ì μ²μ ±μ³ μ ÉÒ ±Êʳ ÒÌ μ μ ( ), Œμ ± 2 Œμ ±μ ± μ Ê É Ò Ê É É ³. Œ.. μ³μ μ μ, Œμ ± ƒê ²μ ˆ... P9-2017-78
P9-2017-78 ˆ.. ƒê ²μ 1,.. Œ ² ±μ 1,..Šμ Í,.. ʳ,.. μ μ 2 ˆ ˆŸ Š Š ˆ ƒ ˆŒ œ ƒ Œ ƒ ƒ Š-Š ˆ 10- Œ ˆ 1 μ Ò É Ì μ²μ ±μ³ μ ÉÒ ±Êʳ ÒÌ μ μ ( ), Œμ ± 2 Œμ ±μ ± μ Ê É Ò Ê É É ³. Œ.. μ³μ μ μ, Œμ ± ƒê ²μ ˆ... P9-2017-78
P ƒ. Œ. ʳ Ö,. É ±, ˆ.. Š Öαμ,. ˆ. ÕÉÕ ±μ,.. ² μ. Š -ŒˆŠ Š : Œ ˆ, œ,
 P13-2013-108 ƒ. Œ. ʳ Ö,. É ±, ˆ.. Š Öαμ,. ˆ. ÕÉÕ ±μ,.. ² μ Š -ŒˆŠ Š : Œ ˆ, œ, Œ ˆ Š ˆ ʳ Ö ƒ. Œ.. P13-2013-108 Š -³ ± μ ±μ : μ ³μ μ É, Ò Ê²ÓÉ ÉÒ, μ ² ³Ò ±É Ò μé μ Ò ÕÉ Ö ËÊ ±Í μ ²Ó Ò μ ³μ μ É Ò É Éμ
P13-2013-108 ƒ. Œ. ʳ Ö,. É ±, ˆ.. Š Öαμ,. ˆ. ÕÉÕ ±μ,.. ² μ Š -ŒˆŠ Š : Œ ˆ, œ, Œ ˆ Š ˆ ʳ Ö ƒ. Œ.. P13-2013-108 Š -³ ± μ ±μ : μ ³μ μ É, Ò Ê²ÓÉ ÉÒ, μ ² ³Ò ±É Ò μé μ Ò ÕÉ Ö ËÊ ±Í μ ²Ó Ò μ ³μ μ É Ò É Éμ
LUO, Hong2Qun LIU, Shao2Pu Ξ LI, Nian2Bing
 2003 61 3, 435 439 ACTA CHIMICA SINICA Vol 61, 2003 No 3, 435 439 2 ΞΞ ( 400715), 2, 2, 2, 3/ 2 2,, 2,, Ne w Methods for the Determination of the Inclusion Constant between Procaine Hydrochloride and 2Cyclodextrin
2003 61 3, 435 439 ACTA CHIMICA SINICA Vol 61, 2003 No 3, 435 439 2 ΞΞ ( 400715), 2, 2, 2, 3/ 2 2,, 2,, Ne w Methods for the Determination of the Inclusion Constant between Procaine Hydrochloride and 2Cyclodextrin
Fused Bis-Benzothiadiazoles as Electron Acceptors
 Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Development of Accurate Quantitative Analytical Methods to Determine Trace Amounts of Carbon, Sulfur, and Oxygen in Steel
 JFE No. 13 26 8 p. 29 34 Development of Accurate Quantitative Analytical Methods to Determine Trace Amounts of Carbon, Sulfur, and Oxygen in Steel YASUHARA Hisao MIYAGI Chiyoko JFE Ph.D. JFE Abstract:
JFE No. 13 26 8 p. 29 34 Development of Accurate Quantitative Analytical Methods to Determine Trace Amounts of Carbon, Sulfur, and Oxygen in Steel YASUHARA Hisao MIYAGI Chiyoko JFE Ph.D. JFE Abstract:
In your answer, you should make clear how evidence for the size of the nucleus follows from your description
 1. Describe briefly one scattering experiment to investigate the size of the nucleus of the atom. Include a description of the properties of the incident radiation which makes it suitable for this experiment.
1. Describe briefly one scattering experiment to investigate the size of the nucleus of the atom. Include a description of the properties of the incident radiation which makes it suitable for this experiment.
Electronic Supplementary Information
 Electronic Supplementary Information Unprecedented Carbon-Carbon Bond Cleavage in Nucleophilic Aziridine Ring Opening Reaction, Efficient Ring Transformation of Aziridines to Imidazolidin-4-ones Jin-Yuan
Electronic Supplementary Information Unprecedented Carbon-Carbon Bond Cleavage in Nucleophilic Aziridine Ring Opening Reaction, Efficient Ring Transformation of Aziridines to Imidazolidin-4-ones Jin-Yuan
Equilibrium pka Table (DMSO Solvent and Reference)
 Equilibrium pka Table (DM olvent and Reference) Copyright ans J. 2016 All Rights Reserved This pka table is on the web: http://www.chem.wisc.edu/areas/reich/pkatable/index.htm Ketones Ar = 26.5 52 19.8
Equilibrium pka Table (DM olvent and Reference) Copyright ans J. 2016 All Rights Reserved This pka table is on the web: http://www.chem.wisc.edu/areas/reich/pkatable/index.htm Ketones Ar = 26.5 52 19.8
ˆŒˆ ˆŸ ˆ Œ ƒ LEPTO/JETSET Ÿ ˆ ƒ
 Ó³ Ÿ. 2014.. 11, º 4(188).. 817Ä827 Š Œ œ ƒˆˆ ˆ ˆŠ ˆŒˆ ˆŸ ˆ Œ ƒ LEPTO/JETSET Ÿ ˆ ƒ Ÿ.. ² ± Ì,. Œ. ŠÊ Íμ,.. μ ± Ö 1, Œ. ƒ. μ ±μ Ñ Ò É ÉÊÉ Ö ÒÌ ² μ, Ê Ò Ê²ÓÉ ÉÒ ³ ÒÌμ μ ÉÖ ²ÒÌ μ μ É μ μ ²Ê μ±μ - Ê Ê μ³ Ö
Ó³ Ÿ. 2014.. 11, º 4(188).. 817Ä827 Š Œ œ ƒˆˆ ˆ ˆŠ ˆŒˆ ˆŸ ˆ Œ ƒ LEPTO/JETSET Ÿ ˆ ƒ Ÿ.. ² ± Ì,. Œ. ŠÊ Íμ,.. μ ± Ö 1, Œ. ƒ. μ ±μ Ñ Ò É ÉÊÉ Ö ÒÌ ² μ, Ê Ò Ê²ÓÉ ÉÒ ³ ÒÌμ μ ÉÖ ²ÒÌ μ μ É μ μ ²Ê μ±μ - Ê Ê μ³ Ö
E#ects of Drying on Bacterial Activity and Iron Formation in Acid Sulfate Soils
 J. Jpn. Soc. Soil Phys. No. 3+, p..3 /1,**, * ** ** E#ects of Drying on Bacterial Activity and Iron Formation in Acid Sulfate Soils Kaoru UENO*, Tadashi ADACHI** and Hajime NARIOKA** * The Graduate School
J. Jpn. Soc. Soil Phys. No. 3+, p..3 /1,**, * ** ** E#ects of Drying on Bacterial Activity and Iron Formation in Acid Sulfate Soils Kaoru UENO*, Tadashi ADACHI** and Hajime NARIOKA** * The Graduate School
Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]
![Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)] Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]](/thumbs/89/98928029.jpg) d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
Supporting Information: Expanding the Armory: Predicting and Tuning Covalent Warhead. Reactivity.
 Supporting Information: Expanding the Armory: Predicting and Tuning Covalent Warhead Reactivity. Richard Lonsdale, Jonathan Burgess, Nicola Colclough, Nichola Davies, Eva M. Lenz, Alexandra L. Orton and
Supporting Information: Expanding the Armory: Predicting and Tuning Covalent Warhead Reactivity. Richard Lonsdale, Jonathan Burgess, Nicola Colclough, Nichola Davies, Eva M. Lenz, Alexandra L. Orton and
SUPPLEMENTARY INFORMATION
 Sensitivity of [Ru(phen) 2 dppz] 2+ Light Switch Emission to Ionic Strength, Temperature, and DNA Sequence and Conformation Andrew W. McKinley, Per Lincoln and Eimer M. Tuite* SUPPLEMENTARY INFORMATION
Sensitivity of [Ru(phen) 2 dppz] 2+ Light Switch Emission to Ionic Strength, Temperature, and DNA Sequence and Conformation Andrew W. McKinley, Per Lincoln and Eimer M. Tuite* SUPPLEMENTARY INFORMATION
Aquinas College. Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET
 Aquinas College Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET Pearson Edexcel Level 3 Advanced Subsidiary and Advanced GCE in Mathematics and Further Mathematics Mathematical
Aquinas College Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET Pearson Edexcel Level 3 Advanced Subsidiary and Advanced GCE in Mathematics and Further Mathematics Mathematical
P Î,.. Š ²³Ò±μ, Œ.. Œ ϱ,.. ʳ ˆ ˆ ˆ ˆŸ ˆŠ Š Š ˆ Ÿ -200
 P9-2011-62. Î,.. Š ²³Ò±μ, Œ.. Œ ϱ,.. ʳ ˆ ˆ ˆ ˆŸ ˆŠ Š Š ˆ Ÿ -200 Î.. P9-2011-62 É μ É μ μ Í μ μ Ö μ ±μ Êα Ê ±μ É ²Ö -200 É ² μ μ Ê É μ É μ Í μ μ Ö Ò ÒÌ μ - ±μ, ±μéμ μ Ö ²Ö É Ö Î ÉÓÕ É ³Ò μ É ± Êα ²
P9-2011-62. Î,.. Š ²³Ò±μ, Œ.. Œ ϱ,.. ʳ ˆ ˆ ˆ ˆŸ ˆŠ Š Š ˆ Ÿ -200 Î.. P9-2011-62 É μ É μ μ Í μ μ Ö μ ±μ Êα Ê ±μ É ²Ö -200 É ² μ μ Ê É μ É μ Í μ μ Ö Ò ÒÌ μ - ±μ, ±μéμ μ Ö ²Ö É Ö Î ÉÓÕ É ³Ò μ É ± Êα ²
NIVEAUX C1&C2 sur l échelle proposée par le Conseil de l Europe
 ΥΠΟΥΡΓΕΙΟ ΠΑΙΔΕΙΑΣ ΚΑΙ ΘΡΗΣΚΕΥΜΑΤΩΝ ΚΡΑΤΙΚΟ ΠΙΣΤΟΠΟΙΗΤΙΚΟ ΓΛΩΣΣΟΜΑΘΕΙΑΣ MINISTÈRE DE L ÉDUCATION ET DES CULTES CERTIFICATION EN LANGUE FRANÇAISE NIVEAUX C1&C2 sur l échelle proposée par le Conseil de l
ΥΠΟΥΡΓΕΙΟ ΠΑΙΔΕΙΑΣ ΚΑΙ ΘΡΗΣΚΕΥΜΑΤΩΝ ΚΡΑΤΙΚΟ ΠΙΣΤΟΠΟΙΗΤΙΚΟ ΓΛΩΣΣΟΜΑΘΕΙΑΣ MINISTÈRE DE L ÉDUCATION ET DES CULTES CERTIFICATION EN LANGUE FRANÇAISE NIVEAUX C1&C2 sur l échelle proposée par le Conseil de l
Studies on the Binding Mechanism of Several Antibiotics and Human Serum Albumin
 2005 63 Vol. 63, 2005 23, 2169 2173 ACTA CHIMICA SINICA No. 23, 2169 2173 a,b a a a *,a ( a 130012) ( b 133002), 26 K A 1.98 10 4, 1.01 10 3, 1.38 10 3, 5.97 10 4 7.15 10 4 L mol 1, n 1.16, 0.86, 1.19,
2005 63 Vol. 63, 2005 23, 2169 2173 ACTA CHIMICA SINICA No. 23, 2169 2173 a,b a a a *,a ( a 130012) ( b 133002), 26 K A 1.98 10 4, 1.01 10 3, 1.38 10 3, 5.97 10 4 7.15 10 4 L mol 1, n 1.16, 0.86, 1.19,
Supporting Information To. Microhydration of caesium compounds: Journal of Molecular Modeling
 Supporting Information To Microhydration of caesium compounds: Cs, CsOH, CsI and Cs 2 I 2 complexes with one to three H 2 O molecules of nuclear safety interest Journal of Molecular Modeling Mária Sudolská
Supporting Information To Microhydration of caesium compounds: Cs, CsOH, CsI and Cs 2 I 2 complexes with one to three H 2 O molecules of nuclear safety interest Journal of Molecular Modeling Mária Sudolská
P ² ± μ. œ Š ƒ Š Ÿƒ ˆŸ Œ œ Œ ƒˆ. μ²μ μ Œ Ê μ μ ±μ Ë Í μ É Í ±μ ³μ²μ (RUSGRAV-13), Œμ ±, Õ Ó 2008.
 P3-2009-104.. ² ± μ ˆ ˆ Š Š ˆ œ Š ƒ Š Ÿƒ ˆŸ Œ œ Œ ƒˆ μ²μ μ Œ Ê μ μ ±μ Ë Í μ É Í ±μ ³μ²μ (RUSGRAV-13), Œμ ±, Õ Ó 2008. ² ± μ.. ²μ μ ± μé±²μ μé ÓÕÉμ μ ±μ μ ±μ ÉÖ μé Ö μ³μðóõ É μ μ ³ ²ÒÌ Ô P3-2009-104 ÓÕÉμ
P3-2009-104.. ² ± μ ˆ ˆ Š Š ˆ œ Š ƒ Š Ÿƒ ˆŸ Œ œ Œ ƒˆ μ²μ μ Œ Ê μ μ ±μ Ë Í μ É Í ±μ ³μ²μ (RUSGRAV-13), Œμ ±, Õ Ó 2008. ² ± μ.. ²μ μ ± μé±²μ μé ÓÕÉμ μ ±μ μ ±μ ÉÖ μé Ö μ³μðóõ É μ μ ³ ²ÒÌ Ô P3-2009-104 ÓÕÉμ
FORMULAS FOR STATISTICS 1
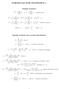 FORMULAS FOR STATISTICS 1 X = 1 n Sample statistics X i or x = 1 n x i (sample mean) S 2 = 1 n 1 s 2 = 1 n 1 (X i X) 2 = 1 n 1 (x i x) 2 = 1 n 1 Xi 2 n n 1 X 2 x 2 i n n 1 x 2 or (sample variance) E(X)
FORMULAS FOR STATISTICS 1 X = 1 n Sample statistics X i or x = 1 n x i (sample mean) S 2 = 1 n 1 s 2 = 1 n 1 (X i X) 2 = 1 n 1 (x i x) 2 = 1 n 1 Xi 2 n n 1 X 2 x 2 i n n 1 x 2 or (sample variance) E(X)
ƒ Š ˆ Šˆ Š Œˆ Šˆ Š ˆŒ PAMELA ˆ AMS-02
 ˆ ˆŠ Œ ˆ ˆ Œ ƒ Ÿ 2017.. 48.. 5.. 582Ä588 œ ˆ Œ ˆ Š Ÿ Š Œ ƒ Š ˆ Šˆ Š Œˆ Šˆ Š ˆŒ PAMELA ˆ AMS-02.. ² ± 1, Š. Œ. ²μͱ 2,.. μ μ³μ²μ 1,. ˆ. Ê 2,.Œ.ƒ ²Ó 2,.. Ê 1,.. Š ²²μ 1, 2,.. ŠÊ Íμ 1,,.. ʱÓÖ μ 1,. ƒ. Œ
ˆ ˆŠ Œ ˆ ˆ Œ ƒ Ÿ 2017.. 48.. 5.. 582Ä588 œ ˆ Œ ˆ Š Ÿ Š Œ ƒ Š ˆ Šˆ Š Œˆ Šˆ Š ˆŒ PAMELA ˆ AMS-02.. ² ± 1, Š. Œ. ²μͱ 2,.. μ μ³μ²μ 1,. ˆ. Ê 2,.Œ.ƒ ²Ó 2,.. Ê 1,.. Š ²²μ 1, 2,.. ŠÊ Íμ 1,,.. ʱÓÖ μ 1,. ƒ. Œ
Nuclear Data Sheets for A = 102
 Nuclear Data Sheets 110 (2009) 1745 1915 www.elsevier.com/locate/nds Nuclear Data Sheets for A = 102 D. DE FRENNE University Ghent Vakgroep Subatomaire en Stralingsfysica Proeftuinstraat 86, B 9000 Gent,
Nuclear Data Sheets 110 (2009) 1745 1915 www.elsevier.com/locate/nds Nuclear Data Sheets for A = 102 D. DE FRENNE University Ghent Vakgroep Subatomaire en Stralingsfysica Proeftuinstraat 86, B 9000 Gent,
