University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution , -.
|
|
|
- ŌἈπολλύων Φραγκούδης
- 5 χρόνια πριν
- Προβολές:
Transcript
1 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution! "#$%&'(#$)*+, -. /, Page 1 of 88
2 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution! "#"$ +, (! #$)*'! "#$%&'013#$$%+! "#$%&' 4 (#$5*' 01 3#$$%'+. 6 &7 78& &4&8: ##+! ; #$%5' (! #$)*' ; ; &<<9+ #+ 97 7&4&8= : #&+ & &8= : #9+ 9+ & &8=&89 +7*4 7 : # &8= : # & 86+97! 867#&4&8=! * #& +7*4&86: #*+ Page of 88
3 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution (! #$)*'(#$%*+ >" %&& $%!" #' #" ( )? 884 &/ & 84+* A! BC46D498 C> E#5 $</#&< 4 &89 F G&H4&86 I#9< I&H4&86 I#9< 84+9 J9 E%< $'*+ ",$%-,&$%"$& Page 3 of 88
4 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution (! #$)*'4#$$9+>, 4#$%%+ - ; ; &<<9+ 4#$$9'(! #$)*+ '.#'+$,& > K/4 +, 4+ ; #& Page 4 of 88
5 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution L4&8 #%$& / (01)34%5 % + << ;" &7,+ /#))5 ;" 97,+ /## ;" /##<6#) ;" /&6&% ;" 4 7,+ << F 4&8,+ /5**)% F ;".; ; &<<9+'F.F #$%%+ G>&$)D ###< /9! ; ;;& E (V) 1.5 Fe 3+ (aq) FeOOH(s) O line Fe + (aq) 0 H line -0.5 Fe 3 O 4 (s) -1 Fe(s) !$," KL4L4&8&$)D! #< /9! ph Page 5 of 88
6 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution! L4&8&$)D #& & #5!86 /,+ K 0+ # <5 < /<5 /# /#5 /& 8 &! &8 9 +! &7,+! &! 84+ & +! + < # & * % ) $ #< ## #& #9 #6 4!$," KL4! L4&8&$)D! #< /9! ## &$)D#< /9! Page 6 of 88
7 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution #9MN!$,"6 KL4L! L4&8&$)D! <<<# E (V) Fe 3+ (aq) Fe + (aq) Mn + (aq) Fe(s) Mn(s) FeO*OH(s) Operating region ph O line H line Page 7 of 88
8 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution 6 #$$$,$#%$#$ #O (#$%5' P&<<#+ /0! K Q0!, (#$%*+ K Q #O ( #$%*+ ( #$%5+ R / S L 884 R/884 ;( #$%*+ 0! ; #$%)+ Page 8 of 88
9 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution &)H&*H ;4 / MN ( #$$#+ / ; 6 7$+$"&$&*+$&& +7*, K,##+ 4 ( #$5*+,. r Fe ( III ) b [ + t O = k Fe( II )] p( O ) k [ Fe( II)] p( ) : #%+ Page 9 of 88
10 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution #6<L#)<, &7,+ <<**L<<$$! +! " #$%&+, [ Fe ] [ O ] 73.9 r exp( ) + = ko : #)+ Fe [ H ] RT k m [ O ] = [ O ] sat : #$+ ko 4 C &7!" #$%&+ Page 10 of 88
11 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution 0 4 &Q8686C&86! 8& #$)6+ T +U D 9L)O 4, Q &<<<+ #$)6+ &7! -; #$)&+T +U ;. r Fe = AO [ Fe ] p( O )exp( ) + A1 [ SO4 )][ Fe ] p( O )exp( ) R T R T : ##< / Page 11 of 88
12 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution,, T +U G 01 3 #$$%+ + ; 5#@O 6%$-%$&&! 8 7$+$9$7*, &7 8& #$)6+ E <#69 O + Q &<<<+, &7 A #$95+,. Page 1 of 88
13 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution &7 78&= 97 78& / : ###+ &7 78& / 7&4 7 = 97 74&8& : ##&+ &7 74&8&= V 784 / : ##9+ &7 784 V = / : ##6+ K,##5+, &7 4*) + r Fe + = k Fe ][ O ] : ##5+ [! " #$%&+ 4 E& ; L 8& &7, &7 78&W 8& &7 : ##*+ 8& &7 7 &7 74&8=& / 748& / : ##%+ 48& / 74 7 =4&8& : ##)+ 4&8&= 4&87X8& : ##$+ &7 78&W 8& &7 : ##*+ &7 74&8W : #&<+ 8& & &8=& / : #&#+ Page 13 of 88
14 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution, #, k k [ Fe ] [ O ] r 3 [ Fe + = : #&&+ ] k 1.16, r k = 1.16 k 1.0 k 1.1 [ Fe + [ H 3 + [ Fe ] k k ] [ O ] ] : #&9+ P K,#&6+ K[ Fe + r[ Fe = : #&6+ 3+ ] 1/ 4 [ H ] ] [ O ] "Y #$$$+! " #$%&+ / M / 8/8 N. 78&B /8/8+ &7 : #&5+ /8/8+ &7 7 B /8/8/+ 67 : #&*+ /8/8/ B &/ /+ &7 : #&%+ Page 14 of 88
15 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution 8/+ &7 7 B &/8+ 67 : #&)+ &/ B84 / 74&87 : #&$ / B&4&8 : #9< & B*4&876 : #9#+ ; #&5 #&* ;;5+ r II Fe II E /( RT ) α [ Fe ] e [ O ] = II 1+ ( α / β )[ Fe ] : #9&+ ARS ; *4$$%&$7$+$ > #9+ Z A#6 [ Page 15 of 88
16 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution O,, F,!$,;! MN N. A k m x DO = = Mass transfer coefficient δ D = Molecular diffusivity of oxygen a O o C &8 & P r δ = Apparent Interface film T8&U δ thickness : #99+ = Interfacial area of all bubbles per unit volume of aqueous phase ; K,#%#)##5#&6#9&+ T8 &U d[ O ] D a \ x 0 dx ([ O ] [ O ] sat ) = DO a o δ DO a o = ([ O ] [ O ]) sat δ = k a ([ O ] [ O ]) O = O o = m o sat Page 16 of 88
17 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution + α O ] : #96+ [ B Page 17 of 88 r Fe! r + = 4r O : #95+ Fe 1! r '[ O ] 4 Fe + = k : #9*+ A 'T +UT4 7 U /KO"+ K, #99+ #9*+ T8&U [ O ] [ O ] k' 1+ k a sat = : #9%+ L ; T8&UBT8&U, #%#)##5#&6#9&+ C #$)9+ m o O ] sat =.5 10 p ( O )exp K i Ci : #9%+ T [
18 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution D B\ ] #9 6 0 D D D 4 7 <<< C89 / <<9 C46 7 <<) / <<% D7 <&9 486 / <#* C7 <&5 84 / <#$! &7 <&% 86 &/ <&)! &7 &7 D <&% Page 18 of 88
19 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution <- ; + K - ; ; - K!(+ ; 86%4&8! 864&8! 8 K! + P(4+ ; + G 4 > G4>+ ; #O)/ #5 ; 5 O - +flowrate. Page 19 of 88
20 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution &9! /, &< O &# $< )< %< *< 5< 6< 9< &< #< << << <5 #< #5 &< &5 9< 95 6< 65 5< FO ;" #5!$," " 444 ;K> ;O; O && Page 0 of 88
21 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution " 4!$," / 7#$ ="# &9, + #O) ;6 + * 4 ( Page 1 of 88
22 University of the Witwatersrand The Oxidation and Precipitation of Iron From a Manganese Sulphate Solution 7#$ %+" P / &5 86%4&8+! 864&8+ &%5 / 9&O! &7 ' 8 ; 4 O &6<, A #< O -4*!88 - & A4*< &< O + 4 Page of 88
23 T-1 T- T-3 V--1 V-- V--3 GA Overhead stirrer Condenser TC ph Meter ph, ToC Needle Valves Rotameter Reactor Vessel Vacuum Pump Hot Plate Sampling Bottle Oxygen cylinder Air cylinder Nitrogen cylinder!$,"6 K / >&9))
24 GA 6!$$&*& &6 /! <&&µ 55 (+P ; #< " 4 "!$,"; ; 8 5< 55 ;, ; ; ;;+? tube 0 >&6)) 0 >
25 ; $ $*&"& >! 8 4 &6;4 *! 8 + > 7#$ &%+"% L&<O 55L)5 >&5))
26 6 6 $ $ &&&%&%,%+ &< O95 O 9#+ &< O 8 TU O <9 <&5 <& <#5 <# <<5 < <<<$ <<<) <<<% <<<* <<<5 T4 7 U <<<6 T! +B<5) O <<<9 T +B<&% O 4B* <<<& 95 O &< O 95 O &< O B%% <<<# < < 5< #<< #5< &<< &5< T47U O!$,"6 K >&*))
27 &6<&<< 9&+ &6< Oxydation by Oxygen time, mins 00 rpm 40 rpm!$,"6 K [FeT], mol/l >&%))
28 6 #+"%$$$*7#$ &"& <#%* O ; 99 [FeT], mol/l Starting ph ~6 [Mn(II)]=0.581 mol/l [Fe(II)]=0.176 mol/l oxygen flowrate=.0 l/min time, mins Run 1 Run Run 3!$,"66 >&)))
29 66 $$$+&#&$$ 66 "$&*&$& G;; 96+/ #$%)+96, / ; / O 8 <*5 <* <55 <5 <65 <6 <95 TU <9 <&5 <& T! +U B%% < 5< #<< #5< &<< &5< 9<< 95<!$,"6; K >&$))
30 oxidation of ferrous sulphate by by oxygen [FeT], mol/l [Mn(II)] [Fe T ] T= 77 oc time, mins [Mn(II)],mol/l!$,"6; K 66 $+&*&$& ; P 5<< 55!;; 4 ;;6+ 9# >9<))
31 G %%! 8 4 A *<< 8 4&8 6 KC T +U O " H +! H + 86 H + 4&8 H + "# <<9& *< %&< <%) 6$) / "& <<)$ *< *$) #<6 5%5 / "9 <<)$ 6&< *69 ##6 / / "6 <#%# *< *)9 #&9 6$& / "5 <#%# &<< **< ##$ / / "* <95# *< *&) #&< )96 / "% <95# &<< *&% ### )&9 &5# ") A <95# &<< *9# ##$ 96% / "$^ <95# &<< *99 #&6 )*% &6) K55 A ^K! 8 / >9#))
32 #$%)+ /! 8 4+ "$ 9#!8 4 / 9# (#$%*'( #$$#+ *< &<< ; ## 9& 6 H + 86H &9 / 884 *&$ / # 5*$ & 84+* 96$ 6<< 9#+ 9&+P >9&))
33 9# "% ") 9# 55 ") "%+ C 666/*&$&&$+&?;+ 95 #< O "% "$+ &<< *<< + ; &<< ( #$%5+;?; 9# *<< >99))
34 Weight % M Fe(II) 95 Run R Temperature ( o C)!$,"6>?; M F e (II) n o M n O 9 5 Weight % ""$ T e m p e ra tu re ( o C )!$,"6>?;! 8 + >96))
35 66; <*&$&&$+& ;_"( + 9*+!$,"6? _"( _"(?; Counts E517A.CAF Fe O 3.H O Position [ Theta] (#$%5#$%*'(#$$#+ 9# $%H ;; >95)) Fe +3 OOH
36 + )5H ;;6+ 6; %# &7$+$ 6; %$$"&%%$ 9% ;;9 (#$$#'Q&<<<'#$)&+ ([FeT]/[FeT]o) Oxidation of ferrous sulphate by oxygen T = 77 oc [Mn(II)] = 0.58 mol/l Starting ph ~ time, mins 0.09 mol/l 0.17mol/l 0.7 mol/l 0.35 mol/l!$,"6@ KT +U >9*))
37 [Fe T ], mol/l GA 6; %7$+*# 9)!$,"61 K T =77 oc [Fe(II)] = 0.35 mol/l [Mn(II)] = 0.58 mol/l Oxygen time, mins Air 6;6 %#" [Fe T ], mol/l T = 77 oc [Fe(II)] = 0.17 mol/l [Mn(II)] = 0.58 mol/l Oxygen time, mins Air 9$ >9%))
38 TU O <6 <9) <9* <96 <9& <9 <&) <&* T +UB<95 O T! +UB<5) O 4J* <&6 <&& <& < 5< #<< #5< &<< &5< 9<< 95< B%% B)5 B55 B**!$,"60 K! "#$%&'013#$$%' #$)9+ 6;; %#' 4 ' 4 >9)))
39 G !8 9#<9## TU O <6 <9) <9* <96 <9& <9 <&) <&* <&6 B%% T! +UB<5)# O T +U B<95 O <&& <& < 5< #<< #5< &<< &5< 9<< 95< 4B95 4B5%!$,"6 K4 >9$))
40 0.009 [H+], mol/l [Fe(II)] = 0.35 mol/l ph 5.7 ph 3.5!$,"6 T4 7 U 4 #$)& time, mins 6;> '=#'+$, 9#& 4 / ""%+ >6<))
41 %<< *<< 5<< 6<< K 0+ 9<< &<< #<< < /#<< /&<< 8 T +UB<95# O B%% < # & * % 4!$,"6 4/4, 4#$$9+,9# CK, ; 97 7B &7 : 9#+ RT ared E = h Eo ln : 9&+ n F aoxd >6#))
42 3+ RT [ Fe ] E = h Eo ln + n F [ Fe ] : 99+ C! " B"O 97 7&4&8B # : 96+, T 97 UBT 884+UT47U 9 OD# : 95+ K,99 [ FeOOH] + E h ' = ln + 3 ln[ ] + 1 [ ] C C H K Fe : 9*+ "B)9#6@O DB $*6)%@O 0B95<DB<<9',9* E h ' = const + ( 0.01 ph) : 9%+ /, KN4+ L<&<# 9#& L<&9,, >6&))
43 G 97 O & #9+ T +UJ< [Fe 3+ ]/[Fe + ] T = 77 oc [Fe(II)] o =0.35 mol/l Oxygen ph!$,"66 6;? $$% $&%&$++ +$,. %% 55 >69))
44 %% #6 / + O <<<<* <<<<5 <<<<6 <<<<9 <<<<& <<<<# < < <<5 <# <#5 <& <&5 <9 <95 <6 TU O %% 55 ;`%%!$,"6; K >66))
45 GA ; - /!<- ; #$$%- +A"$ 99 / ' +,#)! " #$%&' #$)6+' +,6#. &7 7X8&7&4 7 B 97 74&8: 6#+! "#$%&'+ r o α = k [ Fe ] [ O ] [ H ] + β + γ exp( E / RT ) : 6&+ $%& 966T4 7 U +, + α β = k Fe ] [ O ] exp( E / RT ) : 69+ r o [ >65))
46 &7 + d [ Fe ] + α β = ro = k [ Fe ] [ O ] exp( E / RT ) : 66+ dt 965+ ;;; T8&UJT8&U #6+, d [ Fe dt T ] = K [ Fe ] T α [ O ] β sat exp E R T >6*)) : 65+ -'$%#, ab#5)sb<)) B59&@O DB#$%#< ) O <))! " #$%&' #$)6+ #$) #$$%+ "Y #$$$+ #$)6'013#$$%+
47 G,, 65+ 9#6 6#!$,";F, / + O <<<<* <<<<5 <<<<6 <<<<9 <<<<& <<<<# < < <<5 <# <#5 <& <&5 <9 <95 <6 TU O %% 55 ;`%% 8&`%% 8&55 ;`%% >6%))
48 ; - %$&$% +$,%$ K, 65+! " #$%&+ # #9#+ 4 6# "Y #$$$+ / #9#+,;;5 rfe II II E /( RT ) α [ Fe ] e [ O ] = II 1+ ( α / β )[ Fe ] : 6*+ d II E /( RT ) ] α [ Fe ] e = 1+ ( α / β )[ Fe [ O II [ Fe II dt ] ] : 6%+ RSREES,, +! "#$%&'+ RIIS,,8 >6)))
49 , 6%+, 65 "Y #$$$+ RSK B<T UBT U+ 1 [ Fe II 1 II ] [ Fe ], o II 1 α = [ Fe ] ln II t β [ Fe ], o 1 + α [ O t ] exp E RT : 6)+ P 1 [ Fe II 1 II ] [ Fe ], o 1 [ Fe ln t [ Fe ROS α[ O ] exp >6$)) II ] II o E RT 1 t b< 6&
50 !$,"; K;K! "Y #$$$+ FD ##O KO" 69 y = -.769E+00x +.439E-04 R = 9.998E-01 oxygen [Fe(II)] =0.35 mol/l 55 oc oxygen [Fe(II)] =0.351 mol/l 77 oc 1/t (ln([fe II ]/[Fe II ] o )) y = -3.00E+00x E-04 R = 9.963E /t (ln([fe II ]/[Fe II ] o)) /t (1/[Fe II ] - 1/[Fe II ]o) 1/t [1/[Fe II ] - 1/[Fe II ]o) >5<)) y = -.69E+00x E-04 R = 9.99E-01 oxygen mol/l Fe(II) 66 oc /t (ln([fe II ]/[Fe II ] o )) y = -.81E+00x + 1.1E-03 oxygen mol/l Fe(II) 85 oc R = 9.98E /t (ln([fe II ]/[Fe II ] o )) /t (1/[Fe II ] - 1/[Fe II ]o) 1/t (1/[Fe II ] - 1/[Fe II ]o)
51 LnK 1 y = x R = /T (k -1 )!$,";6 ;> "Y #$$$+ 5$<@O G R S K, 6% / ; - II II = ([ Fe ] ) exp [ Fe ] cac SSE : 6$+ T U T U K,6% >5#))
52 ;, K+R S6665 SSE E E E E+09 1.E E+10!$,";; KRK >5&))
53 SSE E E E E+10.00E+10.50E E+10!$,";> KSK R S )<#< $ K,6% 6# ; K> > R S K K 5*5#< $!O $<#< $!O 5)$@O D >59))
54 K, 6%+ 6* 6%?+ Theoritical, [Fe] T, mol/l Experimental, [Fe] T, mol/l!$,;? > >56))
55 frequency Residuals!$,;@ 4 6# 6) %% ;;* >55))
56 !$,";1 [Fe]T, mol/l [Fe T ] o = 0.35 mol/l R =0.96 R =0.94 [Fe T ] o = 0.7 mol/l time, mins Experimental Model Fit Experimental Model Fit >5*)) Oxygen T=77 oc
57 GA > -- 5L&<O ++ &894&8+ ; " & O +,. r Fe 1.58 = [ Fe] T [ O ] sat 53. KJ exp RT mol! O + "Y #$$$+/ "Y #$$$+ 9 II /( RT ) r [ Fe ] e [ O ] = Fe II 9 9 II 1+ ( / )[ Fe ] >5%)) sat (Mol/l. min)
58 4!8 4 Q &<<<+ >5)))
59 -, ' '' ' ' ( )*''(+++,, -'..' /.0 1 /. 1 /. '' ' ''' ' ' '3 CO ''' '3 5-''' '/ '3 5-''' '3 DO 8 :. /.79' 1 / ' '93 '' ; ' '' ; ''<"<;" 4(--, (---, '' '3 5. '' '3 4 = 6 ''' '3 4 >= 6 ''' '3? ' '93 4 = 6 9' ''' '3 47@ " 6 '' ' '3 ## 0 '' # 8 5'.. ' '' >5$))
60 '' # 0 '' A. ' '9 ' ' ' po.79' ''' '3.. '. 3 ' ) ''3# 47@ " 6 ''' '3 5 5 ; 5/ B. ( '. 0'., =3" ' '3' ' C 1'. ' >*<))
61 ! # ;? K 4 #$$5+ D / "A! 88 9 '8 5' '?B#9/&6 & ; ; "&5 $5 5 < '9< '/< &%.65L 5& 9 P #$)$+5 :.''04 6 PD8D8 #$%%+5. '9' '(',/0 P 5 P;" &<<#+ 9 ' ' + > * A; #$)6+ +, 9&#L9< % D #$)5+D! ) (>D">D #$)&+K / L6% $ ( > " #$%5+ S/884 R/&89 5' '.-' '.8 ''9'89 1;)9/)* #< (>" #$%*+", \]9&5L99 ## (@K! ;@ #$)*+-' ' ' 9 K 4 K >*#))
62 #& K P 4? #$$<+ D'' '. -' 5? D ( 8 ( > #$$#+ D / 58 '8'8 " : '9#*5L#%9 #6? ;@(@ #$$<+! K! 8 9 5' 'B&#%L&&) #5?> #$56+ <<;696$/695$ #* 4!! (! ; D #$%%+ ;'.1. '-'. 8 ''9'8 9@@ %+#9%/#6& #% 4> #$$9+ ' '8 ''8 ' 4> c ; #) 4"K(C #$5*+D/8 " ; <1<<>6.6)9%L6)6& #$!!-4;d #$)&+8 + ; (! 889 5' '6B9##/9#) &< F@F ; #$%%+ 97 /864&L86 &/ +)/<8 6C&.*%/%$ &# F- F4@ #$$&+ 1' 1 1 ' ' '" >*&))
63 &&! '9. 1 ' -'.8 ''9'8 9C&6&.6%/5* &9! ' '-' '. 8 9 '?1C%59.#<#/##< &6 CKF4DC #$)9+, 9&#L&% &5 "Y!"Aef@ F! #$$$+ D '9''9 '>;6&&9L6&9& &* "Y!"Aef@ 0 (FK &<<#+ D 5**66%L65& &% "Y!"Aef@ &<<&+L / '9''9' '9; %59/%*< &) >D #$%$+<<5< '909<% L9#< &$ ;4 #$55+8 '9'P> F 9< ;? #$**+#'.-'9' '>>8 9# 0 F@ #$)<+ 1' 1 ' ; > Cd >*9))
64 9& 01"g3D> #$$%+D + 9;; ##9/#&6 99 A P #$) ' & Zhang, W. David, M. and Singh, P. (000). Iron oxidation by SO / O in acidic media, Part II. Effect of copper, Hydrometallurgy, 58, (P-@A; #$)<+8 '9. '.'9' E 9'K#5$#/#*<< 9* 0;? #$* !?/4 C d >*6))
65 ##+$7C $%&#$#% *() 9& <<<!5<<<L&<<<<+, ;; $%$#& ;, 0 F #$)<+ K,'A K'+ K'##+,. v = ( E nl E n l1) / h = E / h : ;##+ 1 λ = c / v = hc / E A > N,/ F, ;, >*5))
66 K ;## K 7hK /hk ; K?!$,"K ;, 7hK+, ''' '/ A, $&$#9&#$+%$. >**))
67 G EH K, E.; P -7 T kbc = e : ;#&+ log10 (1/ T ) = log10 ( I O / I) = abc : ;#9+ log 10 ( I O / I) = A : ;#6+ ;;B P/F + ; ;;+,,, ; A #$)5+ >*%))
68 ,,. *$%" %$A" ;A #$)5+,, +, ;#& +; PKK"/F;! PK". A canalyte c analyte Aanalyte = creference : ;#5+ A reference = creference = A reference A analyte = ; = ; >*)))
69 ; <<* <<5 <<6 <<9 <<& <<# < < 5 #< #5 &< &5 TU!$,";, ; 0 F #$)<+ P FP L / 0 F #$)5+. >*$))
70 4 C ' ;,,, A #$)5+ %& ; ;;,. '.' A-' ' '.' + '.' + '.' >%<))
71 *$%*&$&#& ;; 0 ; ; F- #$$&+! ;; F 5; ; ; A µo &6)9 <& <<*L#5 9%&< <& #L#<< 9)*< <& #5L&<<!! &%$5 <& <<&L5 6<9# <& <5L*< >%#))
72 ; A #$)5+ MN + ; #<<< + #<<<< +, #5#<#5&<' # 9 5 #<<< > 5 5O 9&O # #<<< ; " + ;#& >%&))
73 ##+$7C &"%$"$7$,&, L4&8 #"$$% +74&8B8 +7&4 7,+7& : ;&# &8B986 +7)4 7,+7) : ;&& &8B986 +7&4 7,+7& : ;&9+ & &8B9&89 +7&4 7,+7& : ;&6+ &8 +74&8B&89 +7&4 7,+7& : ;&5+ #"$$% +B &7,+7& : ;&*+ &7,+74&8B8 +7&4 7,+ : ;&%+ 9 &7,+764&8B986 +7)4 7,+7& : ;&)+ & &7,+794&8B&89 +7*4 7,+7& : ;&$+ 97,+7&4&8B ,+ : ;&#<+ #"$$% &7,+B 97,+7 : ;&##+ 97,+764&8B86 &/,+7)4 7,+79 : ;&#&+ &$% 8& B&4&8 : ;	+ &4 7 7&B4& : ;+ ' ' >%9))
74 , ;&#+ ' C,. RT {Re duced state} E = E o ln z F { Oxidised state} A"B#$%) /# D /# B &9<* /#0/#?. o G = z E F = RT ln K AD, ; 4 B& &$)D. o G G o G o + = FeO + H G o Fe G o H O kcal = mol G E o = = V z F C,. + o RT [ FeO][ H ] E = E ln z F [ Fe][ H O] ;, o E = E ph = ph >%6))
75 ;, o E = E ph = ph : ;+ A 4 8&4& ()'*+, ; KL4! L4&8 >#$)*+ #"$$%! +74&8B!8 +7&4 7,+7& : ;+ KB/<%&%L<<5$#4 9!8 +74&8B!986 +7&4 7,+7& : ;&#*+ KB<6*&L<<5$#4 &! &8B9!&89 +7&4 7,+7& : ;&#%+ KB<*)$L<<5$#4!&89 +74&8B&!8& +7&4 7,+7& : ;&#)+ E = ph #"$$%! +B! &7,+7& : ;&#$+ >%5))
76 KB/##%$L<<&$5! &7 +! &7,+74&8B!8 +7&4 7,+ : ;&&<+ F! &7 +B#59#L&4 9! &7,+764&8B!986 +7)4 7,+7& : ;&&#+ KB#)&6L<&9*64L<<))*! &7 + &! &7,+794&8B!&89 +7*4 7,+7& : ;&&&+ KB#669L<#%94L<<5$#! &7 +! &7,+7&4&8B!8& &,+ : ;&&9+ KB#&&)L<###)&4L<<&$5! &7 +!8& +7&4&8B!86 /, & : ;&&6+ KB#*$&L<<%))4L<<#$%!86 / + >%*))
77 ##+$76C " & [Fe]T, mol/l Oxygen T=77 o C Starting [Fe(II)] = mol/l time, mins [Fe]T, mol/l Air T=77 o C Starting [Fe(II)]= mol/l time, mins [Fe]T, mol/l Oxygen T=77 o C Starting [Fe(II)]=0.175 mol/l time, mins [Fe]T, mol/l Air T=77 o C Starting [Fe(II)]=0.175 mol/l time, mins >%%))
78 [Fe]T, mol/l Oxygen T=77 o C Starting [Fe(II)] = 0.71 mol/l [Fe]T, mol/l Air T=77 o C Starting [Fe(II)]=0.175 mol/l time, mins time, mins [Fe]T, mol/l Oxygen T=77 o C Starting [Fe(II)] = mol/l time, mins [Fe]T, mol/l Air T=77 o C Starting[Fe(II)]=0.351mol/L time, mins ;9# ' / >%)))
79 ##+$7;C / $ $%*&$&"#&&B$" "# + P & P ' +,. P & &/ 0+BP86 + 7#$ %+" "G. >%$)) : ;6#+ G <5! P & <#! ;C89 9&H4 #+ ; <6<<< &5</ &+ #<L#5+/ 4 +8 #5< &9* +(P & 9+ P & +
80 P & &< +;P & 6+ G ;C89 ; ; A 5+ G 65##< %"$&! #+! > &+! >7 9+! B! P86 6+B 9+L &+ DP86 K,;6#! 86 &/ B! P86BP86! P86 >)<))
81 ?86 &/ B 86 &/ 86 BP86 86 P86 H86 B86 #<< "%+. # &! < <956# +! > <565 + <59<& +! >7 <*#< + <*<% +! P86 <<*5 + <<%*) + H86 %56 )$6 ;H86 )&6 " BT )&6/%56+O )&6+U^#<< B)5H >)#))
82 ##+$7>C $ $- %$&$%A"$, "Y #$$$+ /, #+ 78&B /8/8^+ &7 : #+ /8/8^+ &7 7 B /8/8/+ 67 : &+ /8/8/ B &/ /+ &7 : 9+ 8/+ &7 7 B &/8+ 67 : 6+ &/ B84 / 74&87& : / B&4&8 : *+ 8 6 &7 78& B*4& : %+ ;#&. : )+ r = r1 = k [ Fe 1 r = r = k II ][ O ] k 1 [( Fe O O*) [( Fe O O*) + ][ Fe II ] k + ] [( Fe O O Fe) 4+ : ] $+ ;// /8/8^+ &7 #B&. k1[ Fe II ][ O ] k 1 [( Fe O O*) + ] = k [( Fe O O*) >)&)) + [ Fe II ] k [( Fe O O Fe) 4+ ]
83 A [( Fe O O*) + k1[ Fe ] = II ][ O ] + k II k [ Fe [( Fe O O Fe) ] + k 1 4+ ] : #<+ ; 9 L 5,/,. & 7 /8/8/ B*4&876 : K, ##+ K [ Fe ] [ H O] III 4 6 ' = 4+ II + [( Fe O O Fe) ][ Fe ] [ H 3O ] 4 : #&+ /8/8/+ 67 [( Fe O O Fe) 4+ ] = [ Fe III K'[ Fe II 4 ] [ H ] [ H O] 3 6 O+ ] 4 : #9+ #9+#<+)+'. II III 4 6 k1[ Fe ][ O ] k [ Fe ] [ H O] r = k 1[ FeII ][ O ] k 1 + II II II + ( k [ Fe ] + k 1) ( k [ Fe ] + k 1) K'[ Fe ] [ H 3O ] 4 : #6+ ;D#B#O/#D&B&O/&, K, %+DBD#D&DN'K, #6+. >)9))
84 A II kk1[ Fe ] [ O ] r = (1 q) : #5+ II 1+ ( k / k )[ Fe ] 1 q = K [ Fe C III [ Fe 4 ] [ H II O] 4 ] [ H O ] 4 : #*+ #B&D#BR /KN#O" &B#BS /KN&O" K, #5+ II a1[ Fe ] [ O ] r = (1 q) : #%+ II 1+ ( a / a )[ Fe ] 1 K, #*+D,= <' #O& ; K, #%+. II ] [ Fe [ O ] E / RT e II α r = 1+ ( α / β )[ Fe ] : #)+ >)6))
85 ##+$7?C-!$ R =0.96 Oxygen T=77 oc [Fe]T, mol/l time, mins Experimental Model Fit [Fe]T, mol/l R =0.90 Oxygen T=77 oc time, mins Experimental Model Fit >)5))
86 0.165 Oxygen 0.16 R =0.90 T=55 C time, mins Experimental Model Fit Air 0.65 R =0.91 T=77 o C time, mins Experimental Model Fit!$,"? K [Fe]T, mol/l [Fe]T, mol/l >)*))
87 [Fe]T, mol/l Air T=77 o C R = time, mins Experimental Model Fit [Fe]T, mol/l R =0.96 Oxygen T= 55 o C time, mins Experimental Model Fit!$,"? ( >)%))
88 +$, The program for the estimation of the parameters is quite long to include them all, therefore sample of it is presented here as an illustration. Experimenta Data C Fexp ( ) T t ( ) T parameters to be estimated A A E R T ( ) starting ferrous concn. saturated oxygen concn C Feo C O A 1 = 0.6 A E = C Fe 0.3 Given 1 A 1. ln C Fe C Fe A 1 A 1. ln C Feo A. 1 C. E O exp. t C Feo A R. T C Fe A 1, A, t Minerr C Fe Calculates the ferrous concentrations for the given n parametrs C Fe C n Fe A 1, A, t n calculates the sum of residual square error by comparing the experimental and calculated values of the ferrous concns. SSE1 n SSE1 = C Fexp n C Fe n C Fe = n C Fexp C Fen t, t 300 n >))))
Mean bond enthalpy Standard enthalpy of formation Bond N H N N N N H O O O
 Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
/&25*+* 24.&6,2(2**02)' 24
 !! "#$ % (33 &' ())**,"-.&/(,01.2(*(33*( ( &,.*(33*( ( 2&/((,*(33*( 24 /&25** 24.&6,2(2**02)' 24 " 0 " ( 78,' 4 (33 72"08 " 2/((,02..2(& (902)' 4 #% 7' 2"8(7 39$:80(& 2/((,* (33; (* 3: &
!! "#$ % (33 &' ())**,"-.&/(,01.2(*(33*( ( &,.*(33*( ( 2&/((,*(33*( 24 /&25** 24.&6,2(2**02)' 24 " 0 " ( 78,' 4 (33 72"08 " 2/((,02..2(& (902)' 4 #% 7' 2"8(7 39$:80(& 2/((,* (33; (* 3: &
REDOX (2) pe as a master variable. C. P. Huang University of Delaware CIEG 632
 REDOX (2) pe as a master variable C. P. Huang University of Delaware CIEG 632 1 8.0 pe as a Master Variable E O,R = E o O,R 2. 303RT log n R O E O, R = E o O, R + 2. 303RT n O log R E R, O = E o R, O 2.
REDOX (2) pe as a master variable C. P. Huang University of Delaware CIEG 632 1 8.0 pe as a Master Variable E O,R = E o O,R 2. 303RT log n R O E O, R = E o O, R + 2. 303RT n O log R E R, O = E o R, O 2.
!"!# ""$ %%"" %$" &" %" "!'! " #$!
 " "" %%"" %" &" %" " " " % ((((( ((( ((((( " %%%% & ) * ((( "* ( + ) (((( (, (() (((((* ( - )((((( )((((((& + )(((((((((( +. ) ) /(((( +( ),(, ((((((( +, 0 )/ (((((+ ++, ((((() & "( %%%%%%%%%%%%%%%%%%%(
" "" %%"" %" &" %" " " " % ((((( ((( ((((( " %%%% & ) * ((( "* ( + ) (((( (, (() (((((* ( - )((((( )((((((& + )(((((((((( +. ) ) /(((( +( ),(, ((((((( +, 0 )/ (((((+ ++, ((((() & "( %%%%%%%%%%%%%%%%%%%(
SIEMENS Squirrel Cage Induction Standard Three-phase Motors
 - SIEMENS Squirrel Cage Induction Standard Three-phase Motors 2 pole 3000 rpm 50Hz Rated current Power Efficiency Rated Ratio Noise Output Frame Speed Weight 3V 400V 415V factor Class 0%Load 75%Load torque
- SIEMENS Squirrel Cage Induction Standard Three-phase Motors 2 pole 3000 rpm 50Hz Rated current Power Efficiency Rated Ratio Noise Output Frame Speed Weight 3V 400V 415V factor Class 0%Load 75%Load torque
DuPont Suva. DuPont. Thermodynamic Properties of. Refrigerant (R-410A) Technical Information. refrigerants T-410A ENG
 Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
..,..,.. ! " # $ % #! & %
 ..,..,.. - -, - 2008 378.146(075.8) -481.28 73 69 69.. - : /..,..,... : - -, 2008. 204. ISBN 5-98298-269-5. - -,, -.,,, -., -. - «- -»,. 378.146(075.8) -481.28 73 -,..,.. ISBN 5-98298-269-5..,..,.., 2008,
..,..,.. - -, - 2008 378.146(075.8) -481.28 73 69 69.. - : /..,..,... : - -, 2008. 204. ISBN 5-98298-269-5. - -,, -.,,, -., -. - «- -»,. 378.146(075.8) -481.28 73 -,..,.. ISBN 5-98298-269-5..,..,.., 2008,
Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]
![Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)] Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]](/thumbs/89/98928029.jpg) d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
Electronic Supplementary Information
 Electronic Supplementary Information The preferred all-gauche conformations in 3-fluoro-1,2-propanediol Laize A. F. Andrade, a Josué M. Silla, a Claudimar J. Duarte, b Roberto Rittner, b Matheus P. Freitas*,a
Electronic Supplementary Information The preferred all-gauche conformations in 3-fluoro-1,2-propanediol Laize A. F. Andrade, a Josué M. Silla, a Claudimar J. Duarte, b Roberto Rittner, b Matheus P. Freitas*,a
DuPont Suva 95 Refrigerant
 Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
! "# $ % $&'& () *+ (,-. / 0 1(,21(,*) (3 4 5 "$ 6, ::: ;"<$& = = 7 + > + 5 $?"# 46(A *( / A 6 ( 1,*1 B"',CD77E *+ *),*,*) F? $G'& 0/ (,.
 ! " #$%&'()' *('+$,&'-. /0 1$23(/%/4. 1$)('%%'($( )/,)$5)/6%6 7$85,-9$(- /0 :/986-$, ;2'$(2$ 1'$-/-$)('')5( /&5&-/ 5(< =(4'($$,'(4 1$%$2/996('25-'/(& ;/0->5,$ 1'$-/%'')$(($/3?$%9'&-/?$( 5(< @6%-'9$
! " #$%&'()' *('+$,&'-. /0 1$23(/%/4. 1$)('%%'($( )/,)$5)/6%6 7$85,-9$(- /0 :/986-$, ;2'$(2$ 1'$-/-$)('')5( /&5&-/ 5(< =(4'($$,'(4 1$%$2/996('25-'/(& ;/0->5,$ 1'$-/%'')$(($/3?$%9'&-/?$( 5(< @6%-'9$
C 1 D 1. AB = a, AD = b, AA1 = c. a, b, c : (1) AC 1 ; : (1) AB + BC + CC1, AC 1 = BC = AD, CC1 = AA 1, AC 1 = a + b + c. (2) BD 1 = BD + DD 1,
 1 1., BD 1 B 1 1 D 1, E F B 1 D 1. B = a, D = b, 1 = c. a, b, c : (1) 1 ; () BD 1 ; () F; D 1 F 1 (4) EF. : (1) B = D, D c b 1 E a B 1 1 = 1, B1 1 = B + B + 1, 1 = a + b + c. () BD 1 = BD + DD 1, BD =
1 1., BD 1 B 1 1 D 1, E F B 1 D 1. B = a, D = b, 1 = c. a, b, c : (1) 1 ; () BD 1 ; () F; D 1 F 1 (4) EF. : (1) B = D, D c b 1 E a B 1 1 = 1, B1 1 = B + B + 1, 1 = a + b + c. () BD 1 = BD + DD 1, BD =
DuPont Suva 95 Refrigerant
 Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
!!" #7 $39 %" (07) ..,..,.. $ 39. ) :. :, «(», «%», «%», «%» «%». & ,. ). & :..,. '.. ( () #*. );..,..'. + (# ).
 1 00 3 !!" 344#7 $39 %" 6181001 63(07) & : ' ( () #* ); ' + (# ) $ 39 ) : : 00 %" 6181001 63(07)!!" 344#7 «(» «%» «%» «%» «%» & ) 4 )&-%/0 +- «)» * «1» «1» «)» ) «(» «%» «%» + ) 30 «%» «%» )1+ / + : +3
1 00 3 !!" 344#7 $39 %" 6181001 63(07) & : ' ( () #* ); ' + (# ) $ 39 ) : : 00 %" 6181001 63(07)!!" 344#7 «(» «%» «%» «%» «%» & ) 4 )&-%/0 +- «)» * «1» «1» «)» ) «(» «%» «%» + ) 30 «%» «%» )1+ / + : +3
Numerical Analysis FMN011
 Numerical Analysis FMN011 Carmen Arévalo Lund University carmen@maths.lth.se Lecture 12 Periodic data A function g has period P if g(x + P ) = g(x) Model: Trigonometric polynomial of order M T M (x) =
Numerical Analysis FMN011 Carmen Arévalo Lund University carmen@maths.lth.se Lecture 12 Periodic data A function g has period P if g(x + P ) = g(x) Model: Trigonometric polynomial of order M T M (x) =
2. Chemical Thermodynamics and Energetics - I
 . Chemical Thermodynamics and Energetics - I 1. Given : Initial Volume ( = 5L dm 3 Final Volume (V = 10L dm 3 ext = 304 cm of Hg Work done W = ext V ext = 304 cm of Hg = 304 atm [... 76cm of Hg = 1 atm]
. Chemical Thermodynamics and Energetics - I 1. Given : Initial Volume ( = 5L dm 3 Final Volume (V = 10L dm 3 ext = 304 cm of Hg Work done W = ext V ext = 304 cm of Hg = 304 atm [... 76cm of Hg = 1 atm]
ΣYΣKEYEΣ ΘEPMIKΩN ΔIEPΓAΣIΩN
 ΠANEΠIΣTHMIO ΘEΣΣAΛIAΣ TMHMA MHXANOΛOΓΩN MHXANIKΩN EPΓAΣTHPIO ΦYΣIKΩN & XHMIKΩN ΔIEPΓAΣIΩN ΣYΣKEYEΣ ΘEPMIKΩN ΔIEPΓAΣIΩN Tεύχος 1ο: Eναλλάκτες μονοφασικής ροής B. Mποντόζογλου BOΛOΣ ΝΟΕΜΒΡΙΟΣ 2013 1. ΠΡΟΚΑΤΑΡΚΤΙΚΟΣ
ΠANEΠIΣTHMIO ΘEΣΣAΛIAΣ TMHMA MHXANOΛOΓΩN MHXANIKΩN EPΓAΣTHPIO ΦYΣIKΩN & XHMIKΩN ΔIEPΓAΣIΩN ΣYΣKEYEΣ ΘEPMIKΩN ΔIEPΓAΣIΩN Tεύχος 1ο: Eναλλάκτες μονοφασικής ροής B. Mποντόζογλου BOΛOΣ ΝΟΕΜΒΡΙΟΣ 2013 1. ΠΡΟΚΑΤΑΡΚΤΙΚΟΣ
Fe II aq Fe III oxide electron transfer and Fe exchange: effect of organic carbon
 Supplementary materials Fe II aq Fe III oxide electron transfer and Fe exchange: effect of organic carbon Timothy Pasakarnis, A Michael L. McCormick, B Gene F. Parkin, A Aaron Thompson C and Michelle M.
Supplementary materials Fe II aq Fe III oxide electron transfer and Fe exchange: effect of organic carbon Timothy Pasakarnis, A Michael L. McCormick, B Gene F. Parkin, A Aaron Thompson C and Michelle M.
2013/2012. m' Z (C) : V= (E): (C) :3,24 m/s. (A) : T= (1-z).g. (D) :4,54 m/s
 ( ) 03/0 - o l P z o M l =.P S. ( ) m' Z l=m m=kg m =,5Kg g=0/kg : : : : Q. (A) : V= (B) : V= () : V= (D) : V= (): : V :Q. (A) :4m/s (B) :0,4 m/s () :5m/s (D) :0,5m/s (): : M T : Q.3 (A) : T=(-z).g (B)
( ) 03/0 - o l P z o M l =.P S. ( ) m' Z l=m m=kg m =,5Kg g=0/kg : : : : Q. (A) : V= (B) : V= () : V= (D) : V= (): : V :Q. (A) :4m/s (B) :0,4 m/s () :5m/s (D) :0,5m/s (): : M T : Q.3 (A) : T=(-z).g (B)
Homework 8 Model Solution Section
 MATH 004 Homework Solution Homework 8 Model Solution Section 14.5 14.6. 14.5. Use the Chain Rule to find dz where z cosx + 4y), x 5t 4, y 1 t. dz dx + dy y sinx + 4y)0t + 4) sinx + 4y) 1t ) 0t + 4t ) sinx
MATH 004 Homework Solution Homework 8 Model Solution Section 14.5 14.6. 14.5. Use the Chain Rule to find dz where z cosx + 4y), x 5t 4, y 1 t. dz dx + dy y sinx + 4y)0t + 4) sinx + 4y) 1t ) 0t + 4t ) sinx
MEASUREMENT OF SURFACE TENSION IN BASE METAL SULFIDE MATTES BY AN IMPROVED SESSILE DROP METHOD
 MEASUREMENT OF SURFACE TENSION IN BASE METAL SULFIDE MATTES BY AN IMPROVED SESSILE DROP METHOD by Joseph Hamuyuni Thesis presented in partial fulfilment of the requirements for the degree of Master of
MEASUREMENT OF SURFACE TENSION IN BASE METAL SULFIDE MATTES BY AN IMPROVED SESSILE DROP METHOD by Joseph Hamuyuni Thesis presented in partial fulfilment of the requirements for the degree of Master of
Parts Manual. Trio Mobile Surgery Platform. Model 1033
 Trio Mobile Surgery Platform Model 1033 Parts Manual For parts or technical assistance: Pour pièces de service ou assistance technique : Für Teile oder technische Unterstützung Anruf: Voor delen of technische
Trio Mobile Surgery Platform Model 1033 Parts Manual For parts or technical assistance: Pour pièces de service ou assistance technique : Für Teile oder technische Unterstützung Anruf: Voor delen of technische
Supporting information. An unusual bifunctional Tb-MOF for highly sensing of Ba 2+ ions and remarkable selectivities of CO 2 /N 2 and CO 2 /CH 4
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information An unusual bifunctional Tb-MOF for highly sensing
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information An unusual bifunctional Tb-MOF for highly sensing
INDEX HOESUNG COIL PARTS
 1. Metal Molding High Current SMD Power Inductor PART NO DEMINSION(mm) Inductance Range Rated DC Current Page MMI 06518 SERIES 6.5 7.1 1.8 1.0uH ~ 4.7uH 9.8A ~ 5.0A 5 MMI 06524 SERIES 6.5 7.1 2.4 0.47uH
1. Metal Molding High Current SMD Power Inductor PART NO DEMINSION(mm) Inductance Range Rated DC Current Page MMI 06518 SERIES 6.5 7.1 1.8 1.0uH ~ 4.7uH 9.8A ~ 5.0A 5 MMI 06524 SERIES 6.5 7.1 2.4 0.47uH
Fused Bis-Benzothiadiazoles as Electron Acceptors
 Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Nickel and Platinum PCP Pincer Complexes Incorporating an Acyclic Diaminoalkyl Central Moiety Connecting Imidazole or Pyrazole Rings
 ickel and Platinum PCP Pincer Complexes Incorporating an Acyclic Diaminoalkyl Central Moiety Connecting Imidazole or Pyrazole Rings Braulio M. Puerta Lombardi, Rudy M. Braun, Chris Gendy, Chia Yun Chang,
ickel and Platinum PCP Pincer Complexes Incorporating an Acyclic Diaminoalkyl Central Moiety Connecting Imidazole or Pyrazole Rings Braulio M. Puerta Lombardi, Rudy M. Braun, Chris Gendy, Chia Yun Chang,
Answers to practice exercises
 Answers to practice exercises Chapter Exercise (Page 5). 9 kg 2. 479 mm. 66 4. 565 5. 225 6. 26 7. 07,70 8. 4 9. 487 0. 70872. $5, Exercise 2 (Page 6). (a) 468 (b) 868 2. (a) 827 (b) 458. (a) 86 kg (b)
Answers to practice exercises Chapter Exercise (Page 5). 9 kg 2. 479 mm. 66 4. 565 5. 225 6. 26 7. 07,70 8. 4 9. 487 0. 70872. $5, Exercise 2 (Page 6). (a) 468 (b) 868 2. (a) 827 (b) 458. (a) 86 kg (b)
A Method of Trajectory Tracking Control for Nonminimum Phase Continuous Time Systems
 IIC-11-8 A Method of Trajectory Tracking Control for Nonminimum Phase Continuous Time Systems Takayuki Shiraishi, iroshi Fujimoto (The University of Tokyo) Abstract The purpose of this paper is achievement
IIC-11-8 A Method of Trajectory Tracking Control for Nonminimum Phase Continuous Time Systems Takayuki Shiraishi, iroshi Fujimoto (The University of Tokyo) Abstract The purpose of this paper is achievement
Conductivity Logging for Thermal Spring Well
 /.,**. 25 +,1- **-- 0/2,,,1- **-- 0/2, +,, +/., +0 /,* Conductivity Logging for Thermal Spring Well Koji SATO +, Tadashi TAKAYA,, Tadashi CHIBA, + Nihon Chika Kenkyuusho Co. Ltd., 0/2,, Hongo, Funabashi,
/.,**. 25 +,1- **-- 0/2,,,1- **-- 0/2, +,, +/., +0 /,* Conductivity Logging for Thermal Spring Well Koji SATO +, Tadashi TAKAYA,, Tadashi CHIBA, + Nihon Chika Kenkyuusho Co. Ltd., 0/2,, Hongo, Funabashi,
(... )..!, ".. (! ) # - $ % % $ & % 2007
 (! ), "! ( ) # $ % & % $ % 007 500 ' 67905:5394!33 : (! ) $, -, * +,'; ), -, *! ' - " #!, $ & % $ ( % %): /!, " ; - : - +', 007 5 ISBN 978-5-7596-0766-3 % % - $, $ &- % $ % %, * $ % - % % # $ $,, % % #-
(! ), "! ( ) # $ % & % $ % 007 500 ' 67905:5394!33 : (! ) $, -, * +,'; ), -, *! ' - " #!, $ & % $ ( % %): /!, " ; - : - +', 007 5 ISBN 978-5-7596-0766-3 % % - $, $ &- % $ % %, * $ % - % % # $ $,, % % #-
C M. V n: n =, (D): V 0,M : V M P = ρ ρ V V. = ρ
 »»...» -300-0 () -300-03 () -3300 3.. 008 4 54. 4. 5 :.. ;.. «....... :. : 008. 37.. :....... 008.. :. :.... 54. 4. 5 5 6 ... : : 3 V mnu V mn AU 3 m () ; N (); N A 6030 3 ; ( ); V 3. : () 0 () 0 3 ()
»»...» -300-0 () -300-03 () -3300 3.. 008 4 54. 4. 5 :.. ;.. «....... :. : 008. 37.. :....... 008.. :. :.... 54. 4. 5 5 6 ... : : 3 V mnu V mn AU 3 m () ; N (); N A 6030 3 ; ( ); V 3. : () 0 () 0 3 ()
Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative cleavage of cyclic ethers. Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative
chlorostibine Iou-Sheng Ke and François P. Gabbai Department of Chemistry, Texas A&M University, College Station, TX
 σ-donor/acceptor confused ligands: The case of a chlorostibine Iou-Sheng Ke and François P. Gabbai Department of Chemistry, Texas A&M University, College Station, TX 77843-3255. *To whom correspondence
σ-donor/acceptor confused ligands: The case of a chlorostibine Iou-Sheng Ke and François P. Gabbai Department of Chemistry, Texas A&M University, College Station, TX 77843-3255. *To whom correspondence
Supplementary Information 1.
 Supplementary Information 1. Fig. S1. Correlations between litter-derived-c and N (percent of initial input) and Al-/Fe- (hydr)oxides dissolved by ammonium oxalate (AO); a) 0 10 cm; b) 10 20 cm; c) 20
Supplementary Information 1. Fig. S1. Correlations between litter-derived-c and N (percent of initial input) and Al-/Fe- (hydr)oxides dissolved by ammonium oxalate (AO); a) 0 10 cm; b) 10 20 cm; c) 20
ΘΕΡΜΟΚΗΠΙΑΚΕΣ ΚΑΛΛΙΕΡΓΕΙΕΣ ΕΚΤΟΣ ΕΔΑΦΟΥΣ ΘΡΕΠΤΙΚΑ ΔΙΑΛΥΜΑΤΑ
 ΘΕΡΜΟΚΗΠΙΑΚΕΣ ΚΑΛΛΙΕΡΓΕΙΕΣ ΕΚΤΟΣ ΕΔΑΦΟΥΣ ΘΡΕΠΤΙΚΑ ΔΙΑΛΥΜΑΤΑ Θρεπτικό διάλυμα Είναι ένα αραιό υδατικό διάλυμα όλων των θρεπτικών στοιχείων που είναι απαραίτητα για τα φυτά, τα οποία βρίσκονται διαλυμένα
ΘΕΡΜΟΚΗΠΙΑΚΕΣ ΚΑΛΛΙΕΡΓΕΙΕΣ ΕΚΤΟΣ ΕΔΑΦΟΥΣ ΘΡΕΠΤΙΚΑ ΔΙΑΛΥΜΑΤΑ Θρεπτικό διάλυμα Είναι ένα αραιό υδατικό διάλυμα όλων των θρεπτικών στοιχείων που είναι απαραίτητα για τα φυτά, τα οποία βρίσκονται διαλυμένα
Model: MTZ22. Data. Cylinder count: 1 Displacement [m³/h]: 6,63 Cylinder capacity [cm³]: 38,1 RPM [min -1 ]: 2900 Weight [kg]: 21 Oil charge [dm³]: 1
![Model: MTZ22. Data. Cylinder count: 1 Displacement [m³/h]: 6,63 Cylinder capacity [cm³]: 38,1 RPM [min -1 ]: 2900 Weight [kg]: 21 Oil charge [dm³]: 1 Model: MTZ22. Data. Cylinder count: 1 Displacement [m³/h]: 6,63 Cylinder capacity [cm³]: 38,1 RPM [min -1 ]: 2900 Weight [kg]: 21 Oil charge [dm³]: 1](/thumbs/90/102819400.jpg) Technical data Data Cylinder count: 1 Displacement [m³/h]: 6,63 Cylinder capacity [cm³]: 38,1 RPM [min -1 ]: 2900 Weight [kg]: 21 Oil charge [dm³]: 1 Oil type: 160PZ Crankcase heater type: PTC 35 W Maximum
Technical data Data Cylinder count: 1 Displacement [m³/h]: 6,63 Cylinder capacity [cm³]: 38,1 RPM [min -1 ]: 2900 Weight [kg]: 21 Oil charge [dm³]: 1 Oil type: 160PZ Crankcase heater type: PTC 35 W Maximum
Simon et al. Supplemental Data Page 1
 Simon et al. Supplemental Data Page 1 Supplemental Data Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction Short
Simon et al. Supplemental Data Page 1 Supplemental Data Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction Short
Microelectronic Circuit Design Third Edition - Part I Solutions to Exercises
 Microelectronic Circuit Design Third Edition - Part I Solutions to Exercises Page 11 CHAPTER 1 V LSB 5.1V 10 bits 5.1V 104bits 5.00 mv V 5.1V MSB.560V 1100010001 9 + 8 + 4 + 0 785 10 V O 786 5.00mV or
Microelectronic Circuit Design Third Edition - Part I Solutions to Exercises Page 11 CHAPTER 1 V LSB 5.1V 10 bits 5.1V 104bits 5.00 mv V 5.1V MSB.560V 1100010001 9 + 8 + 4 + 0 785 10 V O 786 5.00mV or
9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer catalysts
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer
Matrices and Determinants
 Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
 !"#!"!"# $ "# '()!* '+!*, -"*!" $ "#. /01 023 43 56789:3 4 ;8< = 7 >/? 44= 7 @ 90A 98BB8: ;4B0C BD :0 E D:84F3 B8: ;4BG H ;8
!"#!"!"# $ "# '()!* '+!*, -"*!" $ "#. /01 023 43 56789:3 4 ;8< = 7 >/? 44= 7 @ 90A 98BB8: ;4B0C BD :0 E D:84F3 B8: ;4BG H ;8
VBA Microsoft Excel. J. Comput. Chem. Jpn., Vol. 5, No. 1, pp (2006)
 J. Comput. Chem. Jpn., Vol. 5, No. 1, pp. 29 38 (2006) Microsoft Excel, 184-8588 2-24-16 e-mail: yosimura@cc.tuat.ac.jp (Received: July 28, 2005; Accepted for publication: October 24, 2005; Published on
J. Comput. Chem. Jpn., Vol. 5, No. 1, pp. 29 38 (2006) Microsoft Excel, 184-8588 2-24-16 e-mail: yosimura@cc.tuat.ac.jp (Received: July 28, 2005; Accepted for publication: October 24, 2005; Published on
!""#$%!& '% ("#% )'*+, &,!" &, ' %!'"!" &"#"-(5-1-,!&
 !""#$%!& '% ("#% )'*+, "!,'--"!!./%&-'012'& "-')'3"4',"'""-,, &,!" &, 3. - 5 1 ' %!'"!" &"#"-(5-1-,!&,'--1'#". -'!! "--''!,. 3,"'%'%,,-" '4!, 5 #" "!, '%& " 3--& " 4'%! "#!6,%3 "#!3 ",%3 2,-! "#13 '& "#%-,&"#-"-,"-!3&-',,3"
!""#$%!& '% ("#% )'*+, "!,'--"!!./%&-'012'& "-')'3"4',"'""-,, &,!" &, 3. - 5 1 ' %!'"!" &"#"-(5-1-,!&,'--1'#". -'!! "--''!,. 3,"'%'%,,-" '4!, 5 #" "!, '%& " 3--& " 4'%! "#!6,%3 "#!3 ",%3 2,-! "#13 '& "#%-,&"#-"-,"-!3&-',,3"
Appendix B Table of Radionuclides Γ Container 1 Posting Level cm per (mci) mci
 3 H 12.35 Y β Low 80 1 - - Betas: 19 (100%) 11 C 20.38 M β+, EC Low 400 1 5.97 13.7 13 N 9.97 M β+ Low 1 5.97 13.7 Positrons: 960 (99.7%) Gaas: 511 (199.5%) Positrons: 1,199 (99.8%) Gaas: 511 (199.6%)
3 H 12.35 Y β Low 80 1 - - Betas: 19 (100%) 11 C 20.38 M β+, EC Low 400 1 5.97 13.7 13 N 9.97 M β+ Low 1 5.97 13.7 Positrons: 960 (99.7%) Gaas: 511 (199.5%) Positrons: 1,199 (99.8%) Gaas: 511 (199.6%)
! " #! $ % & $ ' ( % & # ) * +, - ) % $!. /. $! $
 [ ] # $ %&$'( %&#) *+,-) %$./.$ $ .$0)(0 1 $( $0 $2 3. 45 6# 27 ) $ # * (.8 %$35 %$'( 9)$- %0)-$) %& ( ),)-)) $)# *) ) ) * $ $ $ %$&) 9 ) )-) %&:: *;$ $$)-) $( $ 0,$# #)$.$0#$ $8 $8 $8 $8,:,:,:,: :: ::
[ ] # $ %&$'( %&#) *+,-) %$./.$ $ .$0)(0 1 $( $0 $2 3. 45 6# 27 ) $ # * (.8 %$35 %$'( 9)$- %0)-$) %& ( ),)-)) $)# *) ) ) * $ $ $ %$&) 9 ) )-) %&:: *;$ $$)-) $( $ 0,$# #)$.$0#$ $8 $8 $8 $8,:,:,:,: :: ::
Eight Examples of Linear and Nonlinear Least Squares
 Eight Examples of Linear and Nonlinear Least Squares CEE 699.4, ME 99.4 Sstem Identification Fall, 21 c Henri P. Gavin, September 2, 21 1 Not polnomial, but linear in parameters. ŷ(t i ; a) = a 1 sin(t
Eight Examples of Linear and Nonlinear Least Squares CEE 699.4, ME 99.4 Sstem Identification Fall, 21 c Henri P. Gavin, September 2, 21 1 Not polnomial, but linear in parameters. ŷ(t i ; a) = a 1 sin(t
HOMEWORK 4 = G. In order to plot the stress versus the stretch we define a normalized stretch:
 HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
Mock Exam 7. 1 Hong Kong Educational Publishing Company. Section A 1. Reference: HKDSE Math M Q2 (a) (1 + kx) n 1M + 1A = (1) =
 Mock Eam 7 Mock Eam 7 Section A. Reference: HKDSE Math M 0 Q (a) ( + k) n nn ( )( k) + nk ( ) + + nn ( ) k + nk + + + A nk... () nn ( ) k... () From (), k...() n Substituting () into (), nn ( ) n 76n 76n
Mock Eam 7 Mock Eam 7 Section A. Reference: HKDSE Math M 0 Q (a) ( + k) n nn ( )( k) + nk ( ) + + nn ( ) k + nk + + + A nk... () nn ( ) k... () From (), k...() n Substituting () into (), nn ( ) n 76n 76n
FORMULAS FOR STATISTICS 1
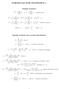 FORMULAS FOR STATISTICS 1 X = 1 n Sample statistics X i or x = 1 n x i (sample mean) S 2 = 1 n 1 s 2 = 1 n 1 (X i X) 2 = 1 n 1 (x i x) 2 = 1 n 1 Xi 2 n n 1 X 2 x 2 i n n 1 x 2 or (sample variance) E(X)
FORMULAS FOR STATISTICS 1 X = 1 n Sample statistics X i or x = 1 n x i (sample mean) S 2 = 1 n 1 s 2 = 1 n 1 (X i X) 2 = 1 n 1 (x i x) 2 = 1 n 1 Xi 2 n n 1 X 2 x 2 i n n 1 x 2 or (sample variance) E(X)
Estimation of grain boundary segregation enthalpy and its role in stable nanocrystalline alloy design
 Supplemental Material for Estimation of grain boundary segregation enthalpy and its role in stable nanocrystalline alloy design By H. A. Murdoch and C.A. Schuh Miedema model RKM model ΔH mix ΔH seg ΔH
Supplemental Material for Estimation of grain boundary segregation enthalpy and its role in stable nanocrystalline alloy design By H. A. Murdoch and C.A. Schuh Miedema model RKM model ΔH mix ΔH seg ΔH
Swirl diffusers, Variable swirl diffusers Swirl diffusers
 , Variable swirl diffusers Swirl diffuser OD-9 Square or round front mask Square or radial deflector arrangement Plastic deflectors Possible volume control damper in spigot Foam sealing on the flange St
, Variable swirl diffusers Swirl diffuser OD-9 Square or round front mask Square or radial deflector arrangement Plastic deflectors Possible volume control damper in spigot Foam sealing on the flange St
w o = R 1 p. (1) R = p =. = 1
 Πανεπιστήµιο Κρήτης - Τµήµα Επιστήµης Υπολογιστών ΗΥ-570: Στατιστική Επεξεργασία Σήµατος 205 ιδάσκων : Α. Μουχτάρης Τριτη Σειρά Ασκήσεων Λύσεις Ασκηση 3. 5.2 (a) From the Wiener-Hopf equation we have:
Πανεπιστήµιο Κρήτης - Τµήµα Επιστήµης Υπολογιστών ΗΥ-570: Στατιστική Επεξεργασία Σήµατος 205 ιδάσκων : Α. Μουχτάρης Τριτη Σειρά Ασκήσεων Λύσεις Ασκηση 3. 5.2 (a) From the Wiener-Hopf equation we have:
Design Method of Ball Mill by Discrete Element Method
 Design Method of Ball Mill by Discrete Element Method Sumitomo Chemical Co., Ltd. Process & Production Technology Center Makio KIMURA Masayuki NARUMI Tomonari KOBAYASHI The grinding rate of gibbsite in
Design Method of Ball Mill by Discrete Element Method Sumitomo Chemical Co., Ltd. Process & Production Technology Center Makio KIMURA Masayuki NARUMI Tomonari KOBAYASHI The grinding rate of gibbsite in
ΜΜ917-Σχεδιασμός Ενεργειακών Συστημάτων
 ΜΜ917-Σχεδιασμός Ενεργειακών Συστημάτων Ψυκτικός Κύκλος Παρουσίαση και περιγραφή τρόπου λειτουργίας των επιμέρους τμημάτων Βιομηχανικής Ψυκτικής Μονάδας ημήτρης Τζιουρτζιούμης ρ. Μηχανολόγος Μηχανικός
ΜΜ917-Σχεδιασμός Ενεργειακών Συστημάτων Ψυκτικός Κύκλος Παρουσίαση και περιγραφή τρόπου λειτουργίας των επιμέρους τμημάτων Βιομηχανικής Ψυκτικής Μονάδας ημήτρης Τζιουρτζιούμης ρ. Μηχανολόγος Μηχανικός
ΤΜΗΜΑ ΦΥΣΙΚΩΝ ΠΟΡΩΝ & ΠΕΡΙΒΑΛΛΟΝΤΟΣ
 ΤΕΧΝΟΛΟΓΙΚΟ ΕΚΠΑΙ ΕΥΤΙΚΟ Ι ΡΥΜΑ ΚΡΗΤΗΣ ΠΑΡΑΡΤΗΜΑ ΧΑΝΙΩΝ ΤΜΗΜΑ ΦΥΣΙΚΩΝ ΠΟΡΩΝ & ΠΕΡΙΒΑΛΛΟΝΤΟΣ ΤΟΜΕΑΣ ΠΕΡΙΒΑΛΛΟΝΤΙΚΗΣ ΤΕΧΝΟΛΟΓΙΑΣ ΕΡΓΑΣΤΗΡΙΟ ΠΕΡΙΒΑΛΛΟΝΤΙΚΗΣ ΧΗΜΕΙΑΣ & ΒΙΟΧΗΜΙΚΩΝ ΙΕΡΓΑΣΙΩΝ ΠΤΥΧΙΑΚΗ ΕΡΓΑΣΙΑ
ΤΕΧΝΟΛΟΓΙΚΟ ΕΚΠΑΙ ΕΥΤΙΚΟ Ι ΡΥΜΑ ΚΡΗΤΗΣ ΠΑΡΑΡΤΗΜΑ ΧΑΝΙΩΝ ΤΜΗΜΑ ΦΥΣΙΚΩΝ ΠΟΡΩΝ & ΠΕΡΙΒΑΛΛΟΝΤΟΣ ΤΟΜΕΑΣ ΠΕΡΙΒΑΛΛΟΝΤΙΚΗΣ ΤΕΧΝΟΛΟΓΙΑΣ ΕΡΓΑΣΤΗΡΙΟ ΠΕΡΙΒΑΛΛΟΝΤΙΚΗΣ ΧΗΜΕΙΑΣ & ΒΙΟΧΗΜΙΚΩΝ ΙΕΡΓΑΣΙΩΝ ΠΤΥΧΙΑΚΗ ΕΡΓΑΣΙΑ
By Futoshi Nagata, Katsuya Inoue, Kozo Shinoda, Shigeru Suzuki and Yoshio Waseda
 13 Green Rust(Cl ) *1, * 1*2, *1, *1, * 1 Synthesis of Green Rust(Cl ) and Characterization of its Oxidation Process By Futoshi Nagata, Katsuya Inoue, Kozo Shinoda, Shigeru Suzuki and Yoshio Waseda Chloride-containing
13 Green Rust(Cl ) *1, * 1*2, *1, *1, * 1 Synthesis of Green Rust(Cl ) and Characterization of its Oxidation Process By Futoshi Nagata, Katsuya Inoue, Kozo Shinoda, Shigeru Suzuki and Yoshio Waseda Chloride-containing
FEATURES APPLICATION PRODUCT T IDENTIFICATION PRODUCT T DIMENSION MAG.LAYERS
 FEATURES RoHS compliant. Super low resistance, ultra high current rating. High performance (I sat) realized by metal dust core. Frequency Range: up to 1MHz. APPLICATION PDA, notebook, desktop, and server
FEATURES RoHS compliant. Super low resistance, ultra high current rating. High performance (I sat) realized by metal dust core. Frequency Range: up to 1MHz. APPLICATION PDA, notebook, desktop, and server
Isotopic and Geochemical Study of Travertine and Hot Springs Occurring Along the Yumoto Fault at North Coast of the Oga Peninsula, Akita Prefecture
 J. Hot Spring Sci. /2 **2 * + + + + + +3 ++ * * + 3 Isotopic and Geochemical Study of Travertine and Hot Springs Occurring Along the Yumoto Fault at North Coast of the Oga Peninsula Akita Prefecture +
J. Hot Spring Sci. /2 **2 * + + + + + +3 ++ * * + 3 Isotopic and Geochemical Study of Travertine and Hot Springs Occurring Along the Yumoto Fault at North Coast of the Oga Peninsula Akita Prefecture +
!"#$%& '!(#)& a<.21c67.<9 /06 :6>/ 54.6: 1. ]1;A76 _F -. /06 4D26.36 <> A.:4D6:6C C4/4 /06 D:43? C</ O=47?6C b*dp 12 :1?6:E /< D6 3:4221N6C 42 D:A6 O=
![!#$%& '!(#)& a<.21c67.<9 /06 :6>/ 54.6: 1. ]1;A76 _F -. /06 4D26.36 <> A.:4D6:6C C4/4 /06 D:43? C</ O=47?6C b*dp 12 :1?6:E /< D6 3:4221N6C 42 D:A6 O= !#$%& '!(#)& a<.21c67.<9 /06 :6>/ 54.6: 1. ]1;A76 _F -. /06 4D26.36 <> A.:4D6:6C C4/4 /06 D:43? C</ O=47?6C b*dp 12 :1?6:E /< D6 3:4221N6C 42 D:A6 O=](/thumbs/72/67457957.jpg) ! " #$% & '( )*+, -. /012 3045/67 8 96 57626./ 4. 4:;74= 69676.36 D426C
! " #$% & '( )*+, -. /012 3045/67 8 96 57626./ 4. 4:;74= 69676.36 D426C
Si + Al Mg Fe + Mn +Ni Ca rim Ca p.f.u
 .6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
.6.5. y = -.4x +.8 R =.9574 y = - x +.14 R =.9788 y = -.4 x +.7 R =.9896 Si + Al Fe + Mn +Ni y =.55 x.36 R =.9988.149.148.147.146.145..88 core rim.144 4 =.6 ±.6 4 =.6 ±.18.84.88 p.f.u..86.76 y = -3.9 x
Heterobimetallic Pd-Sn Catalysis: Michael Addition. Reaction with C-, N-, O-, S- Nucleophiles and In-situ. Diagnostics
 Supporting Information (SI) Heterobimetallic Pd-Sn Catalysis: Michael Addition Reaction with C-, N-, -, S- Nucleophiles and In-situ Diagnostics Debjit Das, a Sanjay Pratihar a,b and Sujit Roy c * a rganometallics
Supporting Information (SI) Heterobimetallic Pd-Sn Catalysis: Michael Addition Reaction with C-, N-, -, S- Nucleophiles and In-situ Diagnostics Debjit Das, a Sanjay Pratihar a,b and Sujit Roy c * a rganometallics
ΚΕΦΑΛΑΙΟ 4 ΘΕΡΜΟΦΥΣΙΚΕΣ Ι ΙΟΤΗΤΕΣ ΤΩΝ ΤΡΟΦΙΜΩΝ
 ΚΕΦΑΛΑΙΟ 4 ΘΕΡΜΟΦΥΣΙΚΕΣ Ι ΙΟΤΗΤΕΣ ΤΩΝ ΤΡΟΦΙΜΩΝ Εισαγωγή Η µελέτη και ο σχεδιασµός όλων των διεργασιών των τροφίµων απαιτούν τη γνώση των θερµοφυσικών ιδιοτήτων τους. Τα τρόφιµα είναι γενικά ανοµοιογενή
ΚΕΦΑΛΑΙΟ 4 ΘΕΡΜΟΦΥΣΙΚΕΣ Ι ΙΟΤΗΤΕΣ ΤΩΝ ΤΡΟΦΙΜΩΝ Εισαγωγή Η µελέτη και ο σχεδιασµός όλων των διεργασιών των τροφίµων απαιτούν τη γνώση των θερµοφυσικών ιδιοτήτων τους. Τα τρόφιµα είναι γενικά ανοµοιογενή
«ΑΝΑΠΣΤΞΖ ΓΠ ΚΑΗ ΥΩΡΗΚΖ ΑΝΑΛΤΖ ΜΔΣΔΩΡΟΛΟΓΗΚΩΝ ΓΔΓΟΜΔΝΩΝ ΣΟΝ ΔΛΛΑΓΗΚΟ ΥΩΡΟ»
 ΓΔΩΠΟΝΗΚΟ ΠΑΝΔΠΗΣΖΜΗΟ ΑΘΖΝΩΝ ΣΜΗΜΑ ΑΞΙΟΠΟΙΗΗ ΦΤΙΚΩΝ ΠΟΡΩΝ & ΓΕΩΡΓΙΚΗ ΜΗΥΑΝΙΚΗ ΣΟΜΕΑ ΕΔΑΦΟΛΟΓΙΑ ΚΑΙ ΓΕΩΡΓΙΚΗ ΥΗΜΕΙΑ ΕΙΔΙΚΕΤΗ: ΕΦΑΡΜΟΓΕ ΣΗ ΓΕΩΠΛΗΡΟΦΟΡΙΚΗ ΣΟΤ ΦΤΙΚΟΤ ΠΟΡΟΤ «ΑΝΑΠΣΤΞΖ ΓΠ ΚΑΗ ΥΩΡΗΚΖ ΑΝΑΛΤΖ ΜΔΣΔΩΡΟΛΟΓΗΚΩΝ
ΓΔΩΠΟΝΗΚΟ ΠΑΝΔΠΗΣΖΜΗΟ ΑΘΖΝΩΝ ΣΜΗΜΑ ΑΞΙΟΠΟΙΗΗ ΦΤΙΚΩΝ ΠΟΡΩΝ & ΓΕΩΡΓΙΚΗ ΜΗΥΑΝΙΚΗ ΣΟΜΕΑ ΕΔΑΦΟΛΟΓΙΑ ΚΑΙ ΓΕΩΡΓΙΚΗ ΥΗΜΕΙΑ ΕΙΔΙΚΕΤΗ: ΕΦΑΡΜΟΓΕ ΣΗ ΓΕΩΠΛΗΡΟΦΟΡΙΚΗ ΣΟΤ ΦΤΙΚΟΤ ΠΟΡΟΤ «ΑΝΑΠΣΤΞΖ ΓΠ ΚΑΗ ΥΩΡΗΚΖ ΑΝΑΛΤΖ ΜΔΣΔΩΡΟΛΟΓΗΚΩΝ
Supporting Information. Experimental section
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Photo-Induced Self-Assembly of Pt(II)-Linked Rings and Cages via the Photolabilization of a Pt(II) Pyridine Bond
 Photo-Induced Self-Assembly of Pt(II)-Linked Rings and Cages via the Photolabilization of a Pt(II) Pyridine Bond Ken-ichi Yamashita, Kei-ichi Sato, Masaki Kawano and Makoto Fujita* Contents; Figure S1.
Photo-Induced Self-Assembly of Pt(II)-Linked Rings and Cages via the Photolabilization of a Pt(II) Pyridine Bond Ken-ichi Yamashita, Kei-ichi Sato, Masaki Kawano and Makoto Fujita* Contents; Figure S1.
ECE Spring Prof. David R. Jackson ECE Dept. Notes 2
 ECE 634 Spring 6 Prof. David R. Jackson ECE Dept. Notes Fields in a Source-Free Region Example: Radiation from an aperture y PEC E t x Aperture Assume the following choice of vector potentials: A F = =
ECE 634 Spring 6 Prof. David R. Jackson ECE Dept. Notes Fields in a Source-Free Region Example: Radiation from an aperture y PEC E t x Aperture Assume the following choice of vector potentials: A F = =
 μ μ μ s t j2 fct T () = a() t e π s t ka t e e j2π fct j2π fcτ0 R() = ( τ0) xt () = α 0 dl () pt ( lt) + wt () l wt () N 2 (0, σ ) Time-Delay Estimation Bias / T c 0.4 0.3 0.2 0.1 0-0.1-0.2-0.3 In-phase
μ μ μ s t j2 fct T () = a() t e π s t ka t e e j2π fct j2π fcτ0 R() = ( τ0) xt () = α 0 dl () pt ( lt) + wt () l wt () N 2 (0, σ ) Time-Delay Estimation Bias / T c 0.4 0.3 0.2 0.1 0-0.1-0.2-0.3 In-phase
,, #,#, %&'(($#(#)&*"& 3,,#!4!4! +&'(#,-$#,./$012 5 # # %, )
 !! "#$%&'%( (%)###**#+!"#$ ',##-.#,,, #,#, /01('/01/'#!2#! %&'(($#(#)&*"& 3,,#!4!4! +&'(#,-$#,./$012 5 # # %, ) 6###+! 4! 4! 4,*!47! 4! (! 8!9%,,#!41! 4! (! 4!5),!(8! 4! (! :!;!(7! (! 4! 4!!8! (! 8! 4!!8(!44!
!! "#$%&'%( (%)###**#+!"#$ ',##-.#,,, #,#, /01('/01/'#!2#! %&'(($#(#)&*"& 3,,#!4!4! +&'(#,-$#,./$012 5 # # %, ) 6###+! 4! 4! 4,*!47! 4! (! 8!9%,,#!41! 4! (! 4!5),!(8! 4! (! :!;!(7! (! 4! 4!!8! (! 8! 4!!8(!44!
Solutions to Exercise Sheet 5
 Solutions to Eercise Sheet 5 jacques@ucsd.edu. Let X and Y be random variables with joint pdf f(, y) = 3y( + y) where and y. Determine each of the following probabilities. Solutions. a. P (X ). b. P (X
Solutions to Eercise Sheet 5 jacques@ucsd.edu. Let X and Y be random variables with joint pdf f(, y) = 3y( + y) where and y. Determine each of the following probabilities. Solutions. a. P (X ). b. P (X
Cycloaddition of Homochiral Dihydroimidazoles: A 1,3-Dipolar Cycloaddition Route to Optically Active Pyrrolo[1,2-a]imidazoles
![Cycloaddition of Homochiral Dihydroimidazoles: A 1,3-Dipolar Cycloaddition Route to Optically Active Pyrrolo[1,2-a]imidazoles Cycloaddition of Homochiral Dihydroimidazoles: A 1,3-Dipolar Cycloaddition Route to Optically Active Pyrrolo[1,2-a]imidazoles](/thumbs/89/100111450.jpg) X-Ray crystallographic data tables for paper: Supplementary Material (ESI) for Organic & Biomolecular Chemistry Cycloaddition of Homochiral Dihydroimidazoles: A 1,3-Dipolar Cycloaddition Route to Optically
X-Ray crystallographic data tables for paper: Supplementary Material (ESI) for Organic & Biomolecular Chemistry Cycloaddition of Homochiral Dihydroimidazoles: A 1,3-Dipolar Cycloaddition Route to Optically
Cite as: Pol Antras, course materials for International Economics I, Spring MIT OpenCourseWare (http://ocw.mit.edu/), Massachusetts
 / / σ/σ σ/σ θ θ θ θ y 1 0.75 0.5 0.25 0 0 0.5 1 1.5 2 θ θ θ x θ θ Φ θ Φ θ Φ π θ /Φ γφ /θ σ θ π θ Φ θ θ Φ θ θ θ θ σ θ / Φ θ θ / Φ / θ / θ Normalized import share: (Xni / Xn) / (XII / XI) 1 0.1 0.01 0.001
/ / σ/σ σ/σ θ θ θ θ y 1 0.75 0.5 0.25 0 0 0.5 1 1.5 2 θ θ θ x θ θ Φ θ Φ θ Φ π θ /Φ γφ /θ σ θ π θ Φ θ θ Φ θ θ θ θ σ θ / Φ θ θ / Φ / θ / θ Normalized import share: (Xni / Xn) / (XII / XI) 1 0.1 0.01 0.001
Ασηπτική Επεξεργασία Των Τροφίµων
 Ασηπτική Επεξεργασία Των Τροφίµων Βασικά Η θέρµανση των τροφίµων εντός δοχείων παρουσιάζει µερικά µειονεκτήµατα, όπως: 1. εν επιτυγχάνεται οµοιόµορφη θέρµανση. 2. Υπάρχει περιορισµός όσον αφορά τη θερµοκρασία
Ασηπτική Επεξεργασία Των Τροφίµων Βασικά Η θέρµανση των τροφίµων εντός δοχείων παρουσιάζει µερικά µειονεκτήµατα, όπως: 1. εν επιτυγχάνεται οµοιόµορφη θέρµανση. 2. Υπάρχει περιορισµός όσον αφορά τη θερµοκρασία
IV. ANHANG 179. Anhang 178
 Anhang 178 IV. ANHANG 179 1. Röntgenstrukturanalysen (Tabellen) 179 1.1. Diastereomer A (Diplomarbeit) 179 1.2. Diastereomer B (Diplomarbeit) 186 1.3. Aldoladdukt 5A 193 1.4. Aldoladdukt 13A 200 1.5. Aldoladdukt
Anhang 178 IV. ANHANG 179 1. Röntgenstrukturanalysen (Tabellen) 179 1.1. Diastereomer A (Diplomarbeit) 179 1.2. Diastereomer B (Diplomarbeit) 186 1.3. Aldoladdukt 5A 193 1.4. Aldoladdukt 13A 200 1.5. Aldoladdukt
r r t r r t t r t P s r t r P s r s r r rs tr t r r t s ss r P s s t r t t tr r r t t r t r r t t s r t rr t Ü rs t 3 r r r 3 rträ 3 röÿ r t
 r t t r t ts r3 s r r t r r t t r t P s r t r P s r s r P s r 1 s r rs tr t r r t s ss r P s s t r t t tr r 2s s r t t r t r r t t s r t rr t Ü rs t 3 r t r 3 s3 Ü rs t 3 r r r 3 rträ 3 röÿ r t r r r rs
r t t r t ts r3 s r r t r r t t r t P s r t r P s r s r P s r 1 s r rs tr t r r t s ss r P s s t r t t tr r 2s s r t t r t r r t t s r t rr t Ü rs t 3 r t r 3 s3 Ü rs t 3 r r r 3 rträ 3 röÿ r t r r r rs
SMD Transient Voltage Suppressors
 SMD Transient Suppressors Feature Full range from 0 to 22 series. form 4 to 60V RMS ; 5.5 to 85Vdc High surge current ability Bidirectional clamping, high energy Fast response time
SMD Transient Suppressors Feature Full range from 0 to 22 series. form 4 to 60V RMS ; 5.5 to 85Vdc High surge current ability Bidirectional clamping, high energy Fast response time
Thi=Τ1. Thο=Τ2. Tci=Τ3. Tco=Τ4. Thm=Τ5. Tcm=Τ6
 1 Τ.Ε.Ι. ΑΘΗΝΑΣ / Σ.ΤΕ.Φ. ΤΜΗΜΑ ΕΝΕΡΓΕΙΑΚΗΣ ΤΕΧΝΟΛΟΓΙΑΣ ΕΡΓΑΣΤΗΡΙΟ ΜΕΤΑΔΟΣΗΣ ΘΕΡΜΟΤΗΤΟΣ Οδός Αγ.Σπυρίδωνος,12210 Αιγάλεω,Αθήνα Τηλ.: 2105385355, email: ptsiling@teiath.gr H ΕΠΙΔΡΑΣΗ ΠΑΡΟΧΗΣ ΟΓΚΟΥ ΣΤΗΝ
1 Τ.Ε.Ι. ΑΘΗΝΑΣ / Σ.ΤΕ.Φ. ΤΜΗΜΑ ΕΝΕΡΓΕΙΑΚΗΣ ΤΕΧΝΟΛΟΓΙΑΣ ΕΡΓΑΣΤΗΡΙΟ ΜΕΤΑΔΟΣΗΣ ΘΕΡΜΟΤΗΤΟΣ Οδός Αγ.Σπυρίδωνος,12210 Αιγάλεω,Αθήνα Τηλ.: 2105385355, email: ptsiling@teiath.gr H ΕΠΙΔΡΑΣΗ ΠΑΡΟΧΗΣ ΟΓΚΟΥ ΣΤΗΝ
ITU-R S (2010/01) &' (
 ITU-R S.52- (200/0) $%!"# &' ( S ITU-R S.52- ii.. (IPR) (ITU-T/ITU-R/ISO/IEC).ITU-R http://www.itu.int/itu-r/go/patents/en. (http://www.itu.int/publ/r-rec/en ) ( ) ( ) BO BR BS BT F M P RA S RS SA SF SM
ITU-R S.52- (200/0) $%!"# &' ( S ITU-R S.52- ii.. (IPR) (ITU-T/ITU-R/ISO/IEC).ITU-R http://www.itu.int/itu-r/go/patents/en. (http://www.itu.int/publ/r-rec/en ) ( ) ( ) BO BR BS BT F M P RA S RS SA SF SM
Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic acids with the assistance of isocyanide
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Cyclopentadienyl iron dicarbonyl (CpFe(CO) 2 ) derivatives
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Cyclopentadienyl iron dicarbonyl (CpFe(CO) 2 ) derivatives
Πρόβλημα 1: Αναζήτηση Ελάχιστης/Μέγιστης Τιμής
 Πρόβλημα 1: Αναζήτηση Ελάχιστης/Μέγιστης Τιμής Να γραφεί πρόγραμμα το οποίο δέχεται ως είσοδο μια ακολουθία S από n (n 40) ακέραιους αριθμούς και επιστρέφει ως έξοδο δύο ακολουθίες από θετικούς ακέραιους
Πρόβλημα 1: Αναζήτηση Ελάχιστης/Μέγιστης Τιμής Να γραφεί πρόγραμμα το οποίο δέχεται ως είσοδο μια ακολουθία S από n (n 40) ακέραιους αριθμούς και επιστρέφει ως έξοδο δύο ακολουθίες από θετικούς ακέραιους
An experimental and theoretical study of the gas phase kinetics of atomic chlorine reactions with CH 3 NH 2, (CH 3 ) 2 NH, and (CH 3 ) 3 N
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
RECIPROCATING COMPRESSOR CALCULATION SHEET ISOTHERMAL COMPRESSION Gas properties, flowrate and conditions. Compressor Calculation Sheet
 RECIPRCATING CMPRESSR CALCULATIN SHEET ISTHERMAL CMPRESSIN Gas properties, flowrate and conditions 1 Gas name Air Item or symbol Quantity Unit Item or symbol Quantity Unit Formula 2 Suction pressure, ps
RECIPRCATING CMPRESSR CALCULATIN SHEET ISTHERMAL CMPRESSIN Gas properties, flowrate and conditions 1 Gas name Air Item or symbol Quantity Unit Item or symbol Quantity Unit Formula 2 Suction pressure, ps
Cable Systems - Postive/Negative Seq Impedance
 Cable Systems - Postive/Negative Seq Impedance Nomenclature: GMD GMR - geometrical mead distance between conductors; depends on construction of the T-line or cable feeder - geometric mean raduius of conductor
Cable Systems - Postive/Negative Seq Impedance Nomenclature: GMD GMR - geometrical mead distance between conductors; depends on construction of the T-line or cable feeder - geometric mean raduius of conductor
1. (a) (5 points) Find the unit tangent and unit normal vectors T and N to the curve. r(t) = 3cost, 4t, 3sint
 1. a) 5 points) Find the unit tangent and unit normal vectors T and N to the curve at the point P, π, rt) cost, t, sint ). b) 5 points) Find curvature of the curve at the point P. Solution: a) r t) sint,,
1. a) 5 points) Find the unit tangent and unit normal vectors T and N to the curve at the point P, π, rt) cost, t, sint ). b) 5 points) Find curvature of the curve at the point P. Solution: a) r t) sint,,
Divergent synthesis of various iminocyclitols from D-ribose
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 205 Divergent synthesis of various iminocyclitols from D-ribose Ramu Petakamsetty,
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 205 Divergent synthesis of various iminocyclitols from D-ribose Ramu Petakamsetty,
!"#$ "%&$ ##%&%'()) *..$ /. 0-1$ )$.'-
 !!" !"# "%& ##%&%',-... /. -1.'- -13-',,'- '-...4 %. -5"'-1.... /..'-1.....-"..'-1.. 78::8
!!" !"# "%& ##%&%',-... /. -1.'- -13-',,'- '-...4 %. -5"'-1.... /..'-1.....-"..'-1.. 78::8
Engineer Reference 1 / / A 35 7/16 X 7 7/8 X 23 5/8 31 1/8 X 21 7/8 X 11 1/4 14 T T 75 R410A oz/ft over 50' 265 / 318 / 388
 Slim Duct, single zone split system SUBMITTAL EH035CAV / UH035CAV Specifications Nominal Capacity Cooling/Heating (Btu/h) 12,000 / 13,600 Cooling (Btu/h) 3,300-14,300 Capacity Range Heating (Btu/h) 3,100-18,000
Slim Duct, single zone split system SUBMITTAL EH035CAV / UH035CAV Specifications Nominal Capacity Cooling/Heating (Btu/h) 12,000 / 13,600 Cooling (Btu/h) 3,300-14,300 Capacity Range Heating (Btu/h) 3,100-18,000
#%" )*& ##+," $ -,!./" %#/%0! %,!
 -!"#$% -&!'"$ & #("$$, #%" )*& ##+," $ -,!./" %#/%0! %,! %!$"#" %!#0&!/" /+#0& 0.00.04. - 3 3,43 5 -, 4 $ $.. 04 ... 3. 6... 6.. #3 7 8... 6.. %9: 3 3 7....3. % 44 8... 6.4. 37; 3,, 443 8... 8.5. $; 3
-!"#$% -&!'"$ & #("$$, #%" )*& ##+," $ -,!./" %#/%0! %,! %!$"#" %!#0&!/" /+#0& 0.00.04. - 3 3,43 5 -, 4 $ $.. 04 ... 3. 6... 6.. #3 7 8... 6.. %9: 3 3 7....3. % 44 8... 6.4. 37; 3,, 443 8... 8.5. $; 3
Solution Series 9. i=1 x i and i=1 x i.
 Lecturer: Prof. Dr. Mete SONER Coordinator: Yilin WANG Solution Series 9 Q1. Let α, β >, the p.d.f. of a beta distribution with parameters α and β is { Γ(α+β) Γ(α)Γ(β) f(x α, β) xα 1 (1 x) β 1 for < x
Lecturer: Prof. Dr. Mete SONER Coordinator: Yilin WANG Solution Series 9 Q1. Let α, β >, the p.d.f. of a beta distribution with parameters α and β is { Γ(α+β) Γ(α)Γ(β) f(x α, β) xα 1 (1 x) β 1 for < x
Aquinas College. Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET
 Aquinas College Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET Pearson Edexcel Level 3 Advanced Subsidiary and Advanced GCE in Mathematics and Further Mathematics Mathematical
Aquinas College Edexcel Mathematical formulae and statistics tables DO NOT WRITE ON THIS BOOKLET Pearson Edexcel Level 3 Advanced Subsidiary and Advanced GCE in Mathematics and Further Mathematics Mathematical
 Problem 7.19 Ignoring reflection at the air soil boundary, if the amplitude of a 3-GHz incident wave is 10 V/m at the surface of a wet soil medium, at what depth will it be down to 1 mv/m? Wet soil is
Problem 7.19 Ignoring reflection at the air soil boundary, if the amplitude of a 3-GHz incident wave is 10 V/m at the surface of a wet soil medium, at what depth will it be down to 1 mv/m? Wet soil is
Enantioselective Organocatalytic Michael Addition of Isorhodanines. to α, β-unsaturated Aldehydes
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
! "#! & "0/! ).#! 71 1&$ -+ #" &> " %+# "1 2$
 "#$" &""'(() *+ , -------------------------------------------------------------------------------------------------------------------. / 0-1 2 $1 " 1 /& 1------------------------------------------------------------------------------------------------------------------------3
"#$" &""'(() *+ , -------------------------------------------------------------------------------------------------------------------. / 0-1 2 $1 " 1 /& 1------------------------------------------------------------------------------------------------------------------------3
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.1245 Non-lattice surface oxygen species implicated in the catalytic partial oxidation of decane to oxygenated aromatics Sivaram Pradhan, Jonathan K. Bartley, Donald Bethell, Albert F.
DOI: 10.1038/NCHEM.1245 Non-lattice surface oxygen species implicated in the catalytic partial oxidation of decane to oxygenated aromatics Sivaram Pradhan, Jonathan K. Bartley, Donald Bethell, Albert F.
Consolidated Drained
 Consolidated Drained q, 8 6 Max. Shear c' =.185 φ' =.8 tan φ' =.69 Deviator, 8 6 6 8 1 1 p', 5 1 15 5 Axial, Symbol Sample ID Depth Test Number Height, in Diameter, in Moisture Content (from Cuttings),
Consolidated Drained q, 8 6 Max. Shear c' =.185 φ' =.8 tan φ' =.69 Deviator, 8 6 6 8 1 1 p', 5 1 15 5 Axial, Symbol Sample ID Depth Test Number Height, in Diameter, in Moisture Content (from Cuttings),
(a b) c = a (b c) e a e = e a = a. a a 1 = a 1 a = e. m+n
 Z 6 D 3 G = {a, b, c,... } G a, b G a b = c c (a b) c = a (b c) e a e = e a = a a a 1 = a 1 a = e Q = {0, ±1, ±2,..., ±n,... } m, n m+n m + 0 = m m + ( m) = 0 Z N = {a n }, n = 1, 2... N N Z N = {1, ω,
Z 6 D 3 G = {a, b, c,... } G a, b G a b = c c (a b) c = a (b c) e a e = e a = a a a 1 = a 1 a = e Q = {0, ±1, ±2,..., ±n,... } m, n m+n m + 0 = m m + ( m) = 0 Z N = {a n }, n = 1, 2... N N Z N = {1, ω,
Supporting Information
 Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
Assalamu `alaikum wr. wb.
 LUMP SUM Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. LUMP SUM Lump sum lump sum lump sum. lump sum fixed price lump sum lump
LUMP SUM Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. Assalamu `alaikum wr. wb. LUMP SUM Wassalamu alaikum wr. wb. LUMP SUM Lump sum lump sum lump sum. lump sum fixed price lump sum lump
Studies on the Binding Mechanism of Several Antibiotics and Human Serum Albumin
 2005 63 Vol. 63, 2005 23, 2169 2173 ACTA CHIMICA SINICA No. 23, 2169 2173 a,b a a a *,a ( a 130012) ( b 133002), 26 K A 1.98 10 4, 1.01 10 3, 1.38 10 3, 5.97 10 4 7.15 10 4 L mol 1, n 1.16, 0.86, 1.19,
2005 63 Vol. 63, 2005 23, 2169 2173 ACTA CHIMICA SINICA No. 23, 2169 2173 a,b a a a *,a ( a 130012) ( b 133002), 26 K A 1.98 10 4, 1.01 10 3, 1.38 10 3, 5.97 10 4 7.15 10 4 L mol 1, n 1.16, 0.86, 1.19,
ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ
 ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΤΜΗΜΑ ΗΛΕΚΤΡΟΛΟΓΩΝ ΜΗΧΑΝΙΚΩΝ & ΜΗΧΑΝΙΚΩΝ ΥΠΟΛΟΓΙΣΤΩΝ ΤΟΜΕΑΣ ΗΛΕΚΤΡΟΝΙΚΗΣ ΜΕΛΕΤΗ ΚΑΙ ΚΑΤΑΣΚΕΥΗ ΙΑΤΑΞΗΣ ΜΕΤΡΗΣΗΣ ΘΕΡΜΟΧΩΡΗΤΙΚΟΤΗΤΑΣ ΣΕ ΜΑΓΝΗΤΙΚΟ ΠΕ ΙΟ ΓΙΑ ΧΑΡΑΚΤΗΡΙΣΜΟ
ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΤΜΗΜΑ ΗΛΕΚΤΡΟΛΟΓΩΝ ΜΗΧΑΝΙΚΩΝ & ΜΗΧΑΝΙΚΩΝ ΥΠΟΛΟΓΙΣΤΩΝ ΤΟΜΕΑΣ ΗΛΕΚΤΡΟΝΙΚΗΣ ΜΕΛΕΤΗ ΚΑΙ ΚΑΤΑΣΚΕΥΗ ΙΑΤΑΞΗΣ ΜΕΤΡΗΣΗΣ ΘΕΡΜΟΧΩΡΗΤΙΚΟΤΗΤΑΣ ΣΕ ΜΑΓΝΗΤΙΚΟ ΠΕ ΙΟ ΓΙΑ ΧΑΡΑΚΤΗΡΙΣΜΟ
