Supporting Information. Martin Srnec, Shaun D. Wong, Megan L. Matthews, Carsten Krebs, J.Martin Bollinger, Edward I. Solomon
|
|
|
- Αναστασούλα Πρίσκα Δάβης
- 7 χρόνια πριν
- Προβολές:
Transcript
1 Electronic Structure of the Ferryl Intermediate in the α- Ketoglutarate Dependent Non-Heme Iron Halogenase SyrB2: Contributions to H-atom Abstraction Reactivity Supporting Information Martin Srnec, Shaun D. Wong, Megan L. Matthews, Carsten Krebs, J.Martin Bollinger, Edward I. Solomon S1
2 Figure S1. A. DFT cluster model of the enzymatic Br Fe IV =O intermediate (taken from Ref. 20 in the main text). B. Truncated model of the Br Fe IV =O structure used in multiconfigurational and multireference calculations. S2
3 Technical details on CASSCF calculations (section 2.6 in the main text) To prevent Fe 3s and three Fe 3p orbitals from mixing with the other orbitals in CASSCF calculations the SUPSYMMETRY keyword of MOLCAS was used. Figure S2. Molecular orbitals of the 16-in-11 active space used in CASSCF/CASPT2 calculations. S3
4 Technical details on RASSCF calculations (section 2.6 in the main text) Technical details on RASSCF calculations (section 2.6 in the main text) The RASSCF active space 26-in-21 used in the study was divided into three subspaces: RAS1 space comprises 16 electrons in 8 orbitals allowing only single and double excitations from this subset of active orbitals; RAS2 space comprises 10 electrons in 8 orbitals with all possible occupations (FCI within RAS2); RAS3 space with 0 electrons in 5 orbitals allowing only single excitations to this subset of active orbitals. The state-average RASSCF were performed over the lowest eleven S = 2 states. The active space orbitals are shown in Figure S2. (SyrB2)Fe IV =O: S4
5 (TMG 3 tren)fe IV =O: Figure S3. Molecular orbitals of the 26-in-21 active space used in RASSCF calculations. NOTE: 4d orbitals were included in RAS3 to account for double-shell effect (see Ref. 32 in the main text). S5
6 Figure S4. A. Temperature dependence of MCD spectral features (I, II and III in Figure 2) measured at ~12100, ~17600 and ~21000 cm -1 and fitted according to Ref. 36 (from the main text) with ZFS D = +7 cm -1 and E/D = B. Schematic depiction of splitting of M s sublevels of an S = 2 species with positive ZFS D parameter in an applied field H. The z axis of the molecule is defined along the Fe oxo bond axis. For z-polarized transitions in MCD, H has a non-vanishing component parallel to the (x,y) plane; at 7 T, the MCD-active M s = 1 sublevel is lowest in energy, so there is MCD intensity at low temperature (temp), but with increasing temp, the population of the M s = 1 sublevel decreases, whereas the populations of the M s = 0, +1 sublevels increase. Because M s = 0 is MCD inactive and M s = +1 produces MCD intensity of the opposite sign, the overall MCD intensity decreases as temp increases. For (x,y)-polarized transitions, H has a non-vanishing component parallel to the z axis; at 7 T, the MCD-inactive M s = 0 sublevel is lowest in energy; as temp increases, the MCD-active M s = 1 sublevel is populated, causing no decrease in MCD intensity, but further increasing temp populates the M s = +1 sublevel and produces MCD intensity of the opposite sign, thereby decreasing overall MCD intensity. Thus, z-pol. transitions start with high MCD intensity at low temp, and decrease with increasing temp, whereas (x;y)-pol. transitions first do not decrease (may even increase) in MCD intensity with increasing temp, then decrease at higher temp. S6
7 Figure S5. Evolution of the two dominant electronic configurations contributing to the CASPT2 wave function characters of the two lowest-energy, low-symmetry split 5 E LF excited states in SyrB2 Br Fe IV =O (the state that is RCP-active in MCD is π-reactive in H-atom abstraction due to the orientation of the π * FMO into the substrate). Other electronic configurations with <10% of weight are not shown. Orbitals associated with the configurations are displayed in Figure 8 (left panel therein). The GS equilibrium and TS Fe O lengths are indicated by a grey bars. S7
8 Figure S6. A. Potential-energy curves (PECs) of the three lowest quintet (S = 2) and ten lowest triplet (S = 1) states along the Fe oxo stretching coordinate. In the vicinity of the ground-state equilibrium, three triplet PECs (denoted as T4, T5 and T6) cross two lowest excited quintet (LCP- and RCP-active) states. In right table, the SOC interactions between these triplets and quintets, calculated as a SOC 3 5 SOC 3 5 SOC 3 = E H x A + E H y A + E H z A, are shown. Note, SOC is strongest between the RCP-active quintet state and the triplet T4 that cross at the ground-state equilibrium. B. Sequence of effects perturbing the LF 5 E state from trigonal-bipyramidal (TBP) C 3 symmetry giving rise to three NIR MCD-active states in the low-symmetry TBP structure of the enzymatic Fe IV =O intermediate. S8
9 Figure S7. Calculated electronic spectra of the SyrB2 Br Fe IV =O (red) and Cl Fe IV =O species (green) using the same level of theory as in Figure 6. Polarization and assignment of transitions are also shown in Figure 6. Orbitals from the active space of RASSCF(26-in-8,5,5) calculations are shown for SyrB2 Br Fe IV =O in Figure S3. The same type of active-space orbitals was used for the SyrB2 Cl Fe IV =O model corresponding to the structure from Figure S1B (the Br ligand is replaced by Cl). S9
10 Figure S8. The potential energy surface of the CT state (root #4 in SA-CASSCF/MS-CASPT2 calculations) of the S = 2 (TMG3tren)Fe IV =O system and SyrB2 Br Fe IV =O intermediate with the oxo group having 0,1 or 2 hydrogen bonds with the second-shell environment. Note: The SA-CASSCF/MS-CASPT2 potential energy surfaces of the three structural models of Fe IV =O were calculated along the Fe O vibration mode of the Br Fe IV =O structure from Figure S1A that was obtained from the BP86/def2-SVP frequency analysis using the G09 program package. S10
11 Figure S9. Geometric and electronic structure parameters for transition states of the π(s Fe =5/2)- controlled H-atom abstraction pathway in both the TMG 3 tren (left) and SyrB2 systems (middle, right). Here, two cluster models for SyrB2 (with and without the second-shell Arginine residue) are presented. Distances are in Å. Table S1. The (activation/reaction) potential energies, enthalpies and free energies of the π(s Fe =5/2)- controlled H-atom abstraction from the native substrate by the (SyrB2)Fe IV =O and (TMG 3 tren)fe IV =O species. The intrinsic reaction barriers are also presented. All values are in kcal mol -1. System E / H / G E 0 / H 0 / G 0 E intr / H intr / G intr (SyrB2)Fe IV =O a 23.1 / 18.9 / / 0.6 / /18.6 / 18.6 (SyrB2)Fe IV =O b 26.5 / 22.5 / / 5.3 / / 19.8 / 22.0 (TMG 3 tren)fe IV =O c 25.3 / 21.6 / / 3.2 / / 20.3 / 21.6 a Within the cluster model from Figure 1D, the second-shell residue Arg 254 appears to destabilize sterically the transition state/product relative to the reactant by ~3-4 kcal mol -1. Here, the DFT results are presented for the cluster model in the absence of the Arg 254 residue. b the Arg-including cluster model of the active site from Figure 1D. c Calculated at the same level of theory as SyrB2 (described in Computational Details) but with a dielectric constant of ε = 35.7 mimicking the solvation effect of acetonitrile (no frznuclei option for vibrational analysis was used). S11
12 XYZ coordinates: Br-Fe IV =O system from Figure S1A O C O O C O C H H Br Fe C N C N N H H H H H H C C N C C N H H H H H C O H H C C N C C N H H H H H C C O O H H H S12
13 H H H H H H H H C H H H C C C C C C H H H H H H O O N C C S C O H H H H C C H H H H H H Truncated Br-Fe IV =O system from Figure S1B O C O C H H Br Fe C N C S13
14 N N H H H H H H C C N C C N H H H H H C C N C C N H H H H H H H H H H H C H H H O O Truncated Cl-Fe IV =O system (in analogy to the Br-Fe IV =O system) O C O C H H Cl Fe C N C N N H S14
15 H H H H H C C N C C N H H H H H C C N C C N H H H H H H H H H H H C H H H O O Cl-bound (SyrB2)Fe IV =O (this includes the second-shell Arg residue) : HAA via π(s FeIII =5/2) channel: Reactant: O C O O C O C H H Cl Fe C N C S15
16 N N H H H H H H H H C C N C C N H H H H H C O H H C C N C C N H H H H H C C O O H H H H H H H H H C H H H O O N C C S S16
17 C O H H H H C H H O H H H H Transition State: O C O O C O C H H Cl Fe C N C N N H H H H H H H H C C N C C N H H H H H C O H H C C S17
18 N C C N H H H H H C C O O H H H H H H H H H C H H H O O N C C S C O H H H H C H H O H H H H Product: O C O O C O C H H Cl S18
19 Fe C N C N N H H H H H H H H C C N C C N H H H H H C O H H C C N C C N H H H H H C C O O H H H H H H H H H C H H H O O S19
20 N C C S C O H H H H C H H O H H H H Cl-bound (SyrB2)Fe IV =O (this model does not include the second-shell Arg residue) : HAA via π(s FeIII =5/2) channel: Reactant: O C O O C O C H H Cl Fe C C N C C N H H H H H C O H H C C N C C N S20
21 H H H H H C C O O H H H H H H H H H C H H H O O N C C S C O H H H H C H H O H H H H Transition State: O C O O C O C H H Cl Fe C C S21
22 N C C N H H H H H C O H H C C N C C N H H H H H C C O O H H H H H H H H H C H H H O O N C C S C O H H H H C H H O H H S22
23 H H Product: O C O O C O C H H Cl Fe C C N C C N H H H H H C O H H C C N C C N H H H H H C C O O H H H H H H H H H C H H H S23
24 O O N C C S C O H H H H C H H O H H H H (TMG 3 tren)fe IV =O self-decay via π(s FeIII =5/2) channel for HAA: Reactant Fe N C H H C H H N C N C H H H C H H H N C H H H C H H H C H H C S24
25 H H N C N C H H H C H H H N C H H H C H H H C H H C H H N C N C H H H C H H H N C H H H C H H H O Transition State: Fe N C H H C S25
26 H H N C N C H H H C H H H N C H H H C H H H C H H C H H N C N C H H H C H H H N C H H H C H H H C H H C H H N C N C H S26
27 H H C H H H N C H H H C H H H O Product Fe N C H H C H H N C N C H H H C H H H N C H H H C H H H C H H C H H N C N C H S27
28 H H C H H H N C H H H C H H H C H H C H H N C N C H H H C H H H N C H H H C H H H O S28
Supporting Information
 Supporting Information rigin of the Regio- and Stereoselectivity of Allylic Substitution of rganocopper Reagents Naohiko Yoshikai, Song-Lin Zhang, and Eiichi Nakamura* Department of Chemistry, The University
Supporting Information rigin of the Regio- and Stereoselectivity of Allylic Substitution of rganocopper Reagents Naohiko Yoshikai, Song-Lin Zhang, and Eiichi Nakamura* Department of Chemistry, The University
Supplementary Materials for. Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides
 Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Electronic Supplementary Information:
 Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Engineering Tunable Single and Dual Optical. Emission from Ru(II)-Polypyridyl Complexes. Through Excited State Design
 Engineering Tunable Single and Dual Optical Emission from Ru(II)-Polypyridyl Complexes Through Excited State Design Supplementary Information Julia Romanova 1, Yousif Sadik 1, M. R. Ranga Prabhath 1,,
Engineering Tunable Single and Dual Optical Emission from Ru(II)-Polypyridyl Complexes Through Excited State Design Supplementary Information Julia Romanova 1, Yousif Sadik 1, M. R. Ranga Prabhath 1,,
of the methanol-dimethylamine complex
 Electronic Supplementary Information for: Fundamental and overtone virational spectroscopy, enthalpy of hydrogen ond formation and equilirium constant determination of the methanol-dimethylamine complex
Electronic Supplementary Information for: Fundamental and overtone virational spectroscopy, enthalpy of hydrogen ond formation and equilirium constant determination of the methanol-dimethylamine complex
Supplementary Information for
 Supplentary Information for Aggregation induced blue-shifted ission molecular picture from QM/MM study Qunyan Wu, a Tian Zhang, a Qian Peng,* b Dong Wang, a and Zhigang Shuai* a a Key Loratory of Organic
Supplentary Information for Aggregation induced blue-shifted ission molecular picture from QM/MM study Qunyan Wu, a Tian Zhang, a Qian Peng,* b Dong Wang, a and Zhigang Shuai* a a Key Loratory of Organic
CHAPTER 25 SOLVING EQUATIONS BY ITERATIVE METHODS
 CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
* To whom correspondence should be addressed.
 SUPPORTING INFORMATION Computational design of a pincer phosphinito vanadium ((OPO)V) propane monoxygenation homogeneous catalyst based on the reduction-coupled oxo activation (ROA) mechanism Ross Fu,
SUPPORTING INFORMATION Computational design of a pincer phosphinito vanadium ((OPO)V) propane monoxygenation homogeneous catalyst based on the reduction-coupled oxo activation (ROA) mechanism Ross Fu,
Electronic Supplementary Information DFT Characterization on the Mechanism of Water Splitting Catalyzed by Single-Ru-substituted Polyoxometalates
 Electronic Supplementary Information DFT Characterization on the Mechanism of Water Splitting Catalyzed by Single-Ru-substituted Polyoxometalates Zhong-Ling Lang, Guo-Chun Yang, Na-Na Ma, Shi-Zheng Wen,
Electronic Supplementary Information DFT Characterization on the Mechanism of Water Splitting Catalyzed by Single-Ru-substituted Polyoxometalates Zhong-Ling Lang, Guo-Chun Yang, Na-Na Ma, Shi-Zheng Wen,
Supporting Information
 SUPPORTING INFORMATION FOR: Trialkylstibine complexes of boron, aluminium, gallium and indium trihalides: synthesis, properties and bonding Victoria K. Greenacre, William Levason and Gillian Reid Chemistry,
SUPPORTING INFORMATION FOR: Trialkylstibine complexes of boron, aluminium, gallium and indium trihalides: synthesis, properties and bonding Victoria K. Greenacre, William Levason and Gillian Reid Chemistry,
Nitric oxide (NO) reactivity studies on mononuclear Iron(II) complexes supported by a tetradentate Schiff base Ligand
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Nitric oxide (NO) reactivity studies on mononuclear Iron(II) complexes supported by a tetradentate
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Nitric oxide (NO) reactivity studies on mononuclear Iron(II) complexes supported by a tetradentate
Solutions to the Schrodinger equation atomic orbitals. Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz
 Solutions to the Schrodinger equation atomic orbitals Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz ybridization Valence Bond Approach to bonding sp 3 (Ψ 2 s + Ψ 2 px + Ψ 2 py + Ψ 2 pz) sp 2 (Ψ 2 s + Ψ 2 px + Ψ 2 py)
Solutions to the Schrodinger equation atomic orbitals Ψ 1 s Ψ 2 s Ψ 2 px Ψ 2 py Ψ 2 pz ybridization Valence Bond Approach to bonding sp 3 (Ψ 2 s + Ψ 2 px + Ψ 2 py + Ψ 2 pz) sp 2 (Ψ 2 s + Ψ 2 px + Ψ 2 py)
Electronic structure and spectroscopy of HBr and HBr +
 Electronic structure and spectroscopy of HBr and HBr + Gabriel J. Vázquez 1 H. P. Liebermann 2 H. Lefebvre Brion 3 1 Universidad Nacional Autónoma de México Cuernavaca México 2 Universität Wuppertal Wuppertal
Electronic structure and spectroscopy of HBr and HBr + Gabriel J. Vázquez 1 H. P. Liebermann 2 H. Lefebvre Brion 3 1 Universidad Nacional Autónoma de México Cuernavaca México 2 Universität Wuppertal Wuppertal
Supporting information. An unusual bifunctional Tb-MOF for highly sensing of Ba 2+ ions and remarkable selectivities of CO 2 /N 2 and CO 2 /CH 4
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information An unusual bifunctional Tb-MOF for highly sensing
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Supporting information An unusual bifunctional Tb-MOF for highly sensing
Supporting Information
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Supporting Information Fluorine Substituent Effect on the Stereochemistry of Catalyzed
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2018 Supporting Information Fluorine Substituent Effect on the Stereochemistry of Catalyzed
An experimental and theoretical study of the gas phase kinetics of atomic chlorine reactions with CH 3 NH 2, (CH 3 ) 2 NH, and (CH 3 ) 3 N
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
(1) Describe the process by which mercury atoms become excited in a fluorescent tube (3)
 Q1. (a) A fluorescent tube is filled with mercury vapour at low pressure. In order to emit electromagnetic radiation the mercury atoms must first be excited. (i) What is meant by an excited atom? (1) (ii)
Q1. (a) A fluorescent tube is filled with mercury vapour at low pressure. In order to emit electromagnetic radiation the mercury atoms must first be excited. (i) What is meant by an excited atom? (1) (ii)
Photo-Induced Self-Assembly of Pt(II)-Linked Rings and Cages via the Photolabilization of a Pt(II) Pyridine Bond
 Photo-Induced Self-Assembly of Pt(II)-Linked Rings and Cages via the Photolabilization of a Pt(II) Pyridine Bond Ken-ichi Yamashita, Kei-ichi Sato, Masaki Kawano and Makoto Fujita* Contents; Figure S1.
Photo-Induced Self-Assembly of Pt(II)-Linked Rings and Cages via the Photolabilization of a Pt(II) Pyridine Bond Ken-ichi Yamashita, Kei-ichi Sato, Masaki Kawano and Makoto Fujita* Contents; Figure S1.
Table of Contents 1 Supplementary Data MCD
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
Supporting Information
 Supporting Information On the Ambiguity of 1,3,2-Benzodiazaboroles as Donor/Acceptor unctionalities in Luminescent Molecules Lothar Weber *[a], Johannes Halama [a], Kenny Hanke [a], Lena Böhling [a], Andreas
Supporting Information On the Ambiguity of 1,3,2-Benzodiazaboroles as Donor/Acceptor unctionalities in Luminescent Molecules Lothar Weber *[a], Johannes Halama [a], Kenny Hanke [a], Lena Böhling [a], Andreas
EE512: Error Control Coding
 EE512: Error Control Coding Solution for Assignment on Finite Fields February 16, 2007 1. (a) Addition and Multiplication tables for GF (5) and GF (7) are shown in Tables 1 and 2. + 0 1 2 3 4 0 0 1 2 3
EE512: Error Control Coding Solution for Assignment on Finite Fields February 16, 2007 1. (a) Addition and Multiplication tables for GF (5) and GF (7) are shown in Tables 1 and 2. + 0 1 2 3 4 0 0 1 2 3
[1] P Q. Fig. 3.1
![[1] P Q. Fig. 3.1 [1] P Q. Fig. 3.1](/thumbs/79/80362156.jpg) 1 (a) Define resistance....... [1] (b) The smallest conductor within a computer processing chip can be represented as a rectangular block that is one atom high, four atoms wide and twenty atoms long. One
1 (a) Define resistance....... [1] (b) The smallest conductor within a computer processing chip can be represented as a rectangular block that is one atom high, four atoms wide and twenty atoms long. One
ELECTRONIC SUPPORTING INFORMATION
 ELECTRONIC SUPPORTING INFORMATION Spectroscopic signatures of the carbon buckyonions C 60 @C 180 and C 60 @C 240 : a dispersion-corrected DFT study. Girolamo Casella, a Alessandro Bagno a and Giacomo Saielli*
ELECTRONIC SUPPORTING INFORMATION Spectroscopic signatures of the carbon buckyonions C 60 @C 180 and C 60 @C 240 : a dispersion-corrected DFT study. Girolamo Casella, a Alessandro Bagno a and Giacomo Saielli*
10-π-electron arenes à la carte: Structure. Sr, Ba; n = 6-8) complexes
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2016 Supporting information 10-π-electron arenes à la carte: Structure and Bonding of
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2016 Supporting information 10-π-electron arenes à la carte: Structure and Bonding of
C.S. 430 Assignment 6, Sample Solutions
 C.S. 430 Assignment 6, Sample Solutions Paul Liu November 15, 2007 Note that these are sample solutions only; in many cases there were many acceptable answers. 1 Reynolds Problem 10.1 1.1 Normal-order
C.S. 430 Assignment 6, Sample Solutions Paul Liu November 15, 2007 Note that these are sample solutions only; in many cases there were many acceptable answers. 1 Reynolds Problem 10.1 1.1 Normal-order
Supporting Information. Generation Response. Physics & Chemistry of CAS, 40-1 South Beijing Road, Urumqi , China. China , USA
 Supporting Information Pb 3 B 6 O 11 F 2 : A First Noncentrocentric Lead Fluoroborate with Large Second Harmonic Generation Response Hongyi Li, a Hongping Wu, a * Xin Su, a Hongwei Yu, a,b Shilie Pan,
Supporting Information Pb 3 B 6 O 11 F 2 : A First Noncentrocentric Lead Fluoroborate with Large Second Harmonic Generation Response Hongyi Li, a Hongping Wu, a * Xin Su, a Hongwei Yu, a,b Shilie Pan,
Approximation of distance between locations on earth given by latitude and longitude
 Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
the total number of electrons passing through the lamp.
 1. A 12 V 36 W lamp is lit to normal brightness using a 12 V car battery of negligible internal resistance. The lamp is switched on for one hour (3600 s). For the time of 1 hour, calculate (i) the energy
1. A 12 V 36 W lamp is lit to normal brightness using a 12 V car battery of negligible internal resistance. The lamp is switched on for one hour (3600 s). For the time of 1 hour, calculate (i) the energy
Section 8.3 Trigonometric Equations
 99 Section 8. Trigonometric Equations Objective 1: Solve Equations Involving One Trigonometric Function. In this section and the next, we will exple how to solving equations involving trigonometric functions.
99 Section 8. Trigonometric Equations Objective 1: Solve Equations Involving One Trigonometric Function. In this section and the next, we will exple how to solving equations involving trigonometric functions.
Dipole-Guided Electron Capture Causes Abnormal Dissociations of Phosphorylated Pentapeptides
 Supplementary Material for Dipole-Guided Electron Capture Causes Abnormal Dissociations of Phosphorylated Pentapeptides Christopher L. Moss, a Thomas W. Chung, a Jean A. Wyer, b Steen Brøndsted Nielsen,
Supplementary Material for Dipole-Guided Electron Capture Causes Abnormal Dissociations of Phosphorylated Pentapeptides Christopher L. Moss, a Thomas W. Chung, a Jean A. Wyer, b Steen Brøndsted Nielsen,
Derivation of Optical-Bloch Equations
 Appendix C Derivation of Optical-Bloch Equations In this appendix the optical-bloch equations that give the populations and coherences for an idealized three-level Λ system, Fig. 3. on page 47, will be
Appendix C Derivation of Optical-Bloch Equations In this appendix the optical-bloch equations that give the populations and coherences for an idealized three-level Λ system, Fig. 3. on page 47, will be
Finite Field Problems: Solutions
 Finite Field Problems: Solutions 1. Let f = x 2 +1 Z 11 [x] and let F = Z 11 [x]/(f), a field. Let Solution: F =11 2 = 121, so F = 121 1 = 120. The possible orders are the divisors of 120. Solution: The
Finite Field Problems: Solutions 1. Let f = x 2 +1 Z 11 [x] and let F = Z 11 [x]/(f), a field. Let Solution: F =11 2 = 121, so F = 121 1 = 120. The possible orders are the divisors of 120. Solution: The
3.4 SUM AND DIFFERENCE FORMULAS. NOTE: cos(α+β) cos α + cos β cos(α-β) cos α -cos β
 3.4 SUM AND DIFFERENCE FORMULAS Page Theorem cos(αβ cos α cos β -sin α cos(α-β cos α cos β sin α NOTE: cos(αβ cos α cos β cos(α-β cos α -cos β Proof of cos(α-β cos α cos β sin α Let s use a unit circle
3.4 SUM AND DIFFERENCE FORMULAS Page Theorem cos(αβ cos α cos β -sin α cos(α-β cos α cos β sin α NOTE: cos(αβ cos α cos β cos(α-β cos α -cos β Proof of cos(α-β cos α cos β sin α Let s use a unit circle
Homework 8 Model Solution Section
 MATH 004 Homework Solution Homework 8 Model Solution Section 14.5 14.6. 14.5. Use the Chain Rule to find dz where z cosx + 4y), x 5t 4, y 1 t. dz dx + dy y sinx + 4y)0t + 4) sinx + 4y) 1t ) 0t + 4t ) sinx
MATH 004 Homework Solution Homework 8 Model Solution Section 14.5 14.6. 14.5. Use the Chain Rule to find dz where z cosx + 4y), x 5t 4, y 1 t. dz dx + dy y sinx + 4y)0t + 4) sinx + 4y) 1t ) 0t + 4t ) sinx
Hydrogen Sorption Efficiency of Titanium Decorated Calix[4]pyrroles
![Hydrogen Sorption Efficiency of Titanium Decorated Calix[4]pyrroles Hydrogen Sorption Efficiency of Titanium Decorated Calix[4]pyrroles](/thumbs/84/89186739.jpg) Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 Hydrogen Sorption Efficiency of Titanium Decorated Calix[4]pyrroles Sandeep Kumar,
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 Hydrogen Sorption Efficiency of Titanium Decorated Calix[4]pyrroles Sandeep Kumar,
Areas and Lengths in Polar Coordinates
 Kiryl Tsishchanka Areas and Lengths in Polar Coordinates In this section we develop the formula for the area of a region whose boundary is given by a polar equation. We need to use the formula for the
Kiryl Tsishchanka Areas and Lengths in Polar Coordinates In this section we develop the formula for the area of a region whose boundary is given by a polar equation. We need to use the formula for the
Manuscript submitted to the Journal of the American Society for Mass Spectrometry, September 2011.
 The Early Life of a Peptide Cation-Radical. Ground and Excited-State Trajectories of Electron-Based Peptide Dissociations During the First 330 Femtoseconds Christopher L. Moss, Wenkel Liang, Xiaosong Li,*
The Early Life of a Peptide Cation-Radical. Ground and Excited-State Trajectories of Electron-Based Peptide Dissociations During the First 330 Femtoseconds Christopher L. Moss, Wenkel Liang, Xiaosong Li,*
Supporting Information
 Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
Supporting Information Aluminum Complexes of N 2 O 2 3 Formazanate Ligands Supported by Phosphine Oxide Donors Ryan R. Maar, Amir Rabiee Kenaree, Ruizhong Zhang, Yichen Tao, Benjamin D. Katzman, Viktor
2 Composition. Invertible Mappings
 Arkansas Tech University MATH 4033: Elementary Modern Algebra Dr. Marcel B. Finan Composition. Invertible Mappings In this section we discuss two procedures for creating new mappings from old ones, namely,
Arkansas Tech University MATH 4033: Elementary Modern Algebra Dr. Marcel B. Finan Composition. Invertible Mappings In this section we discuss two procedures for creating new mappings from old ones, namely,
Mean bond enthalpy Standard enthalpy of formation Bond N H N N N N H O O O
 Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) CPh 3 as a functional group in P-heterocyclic
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) CPh 3 as a functional group in P-heterocyclic
Figure 3 Three observations (Vp, Vs and density isosurfaces) intersecting in the PLF space. Solutions exist at the two indicated points.
 φ φ φ φ Figure 1 Resampling of a rock-physics model from velocity-porosity to lithology-porosity space. C i are model results for various clay contents. φ ρ ρ δ Figure 2 Bulk modulus constraint cube in
φ φ φ φ Figure 1 Resampling of a rock-physics model from velocity-porosity to lithology-porosity space. C i are model results for various clay contents. φ ρ ρ δ Figure 2 Bulk modulus constraint cube in
derivation of the Laplacian from rectangular to spherical coordinates
 derivation of the Laplacian from rectangular to spherical coordinates swapnizzle 03-03- :5:43 We begin by recognizing the familiar conversion from rectangular to spherical coordinates (note that φ is used
derivation of the Laplacian from rectangular to spherical coordinates swapnizzle 03-03- :5:43 We begin by recognizing the familiar conversion from rectangular to spherical coordinates (note that φ is used
CRASH COURSE IN PRECALCULUS
 CRASH COURSE IN PRECALCULUS Shiah-Sen Wang The graphs are prepared by Chien-Lun Lai Based on : Precalculus: Mathematics for Calculus by J. Stuwart, L. Redin & S. Watson, 6th edition, 01, Brooks/Cole Chapter
CRASH COURSE IN PRECALCULUS Shiah-Sen Wang The graphs are prepared by Chien-Lun Lai Based on : Precalculus: Mathematics for Calculus by J. Stuwart, L. Redin & S. Watson, 6th edition, 01, Brooks/Cole Chapter
Fused Bis-Benzothiadiazoles as Electron Acceptors
 Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Fused Bis-Benzothiadiazoles as Electron Acceptors Debin Xia, a,b Xiao-Ye Wang, b Xin Guo, c Martin Baumgarten,*,b Mengmeng Li, b and Klaus Müllen*,b a MIIT Key Laboratory of ritical Materials Technology
Απόκριση σε Μοναδιαία Ωστική Δύναμη (Unit Impulse) Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο. Απόστολος Σ.
 Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο The time integral of a force is referred to as impulse, is determined by and is obtained from: Newton s 2 nd Law of motion states that the action
Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο The time integral of a force is referred to as impulse, is determined by and is obtained from: Newton s 2 nd Law of motion states that the action
DESIGN OF MACHINERY SOLUTION MANUAL h in h 4 0.
 DESIGN OF MACHINERY SOLUTION MANUAL -7-1! PROBLEM -7 Statement: Design a double-dwell cam to move a follower from to 25 6, dwell for 12, fall 25 and dwell for the remader The total cycle must take 4 sec
DESIGN OF MACHINERY SOLUTION MANUAL -7-1! PROBLEM -7 Statement: Design a double-dwell cam to move a follower from to 25 6, dwell for 12, fall 25 and dwell for the remader The total cycle must take 4 sec
Phys460.nb Solution for the t-dependent Schrodinger s equation How did we find the solution? (not required)
 Phys460.nb 81 ψ n (t) is still the (same) eigenstate of H But for tdependent H. The answer is NO. 5.5.5. Solution for the tdependent Schrodinger s equation If we assume that at time t 0, the electron starts
Phys460.nb 81 ψ n (t) is still the (same) eigenstate of H But for tdependent H. The answer is NO. 5.5.5. Solution for the tdependent Schrodinger s equation If we assume that at time t 0, the electron starts
Supporting Information for: electron ligands: Complex formation, oxidation and
 Supporting Information for: The diverse reactions of PhI(OTf) 2 with common 2- electron ligands: Complex formation, oxidation and oxidative coupling Thomas P. Pell, Shannon A. Couchman, Sara Ibrahim, David
Supporting Information for: The diverse reactions of PhI(OTf) 2 with common 2- electron ligands: Complex formation, oxidation and oxidative coupling Thomas P. Pell, Shannon A. Couchman, Sara Ibrahim, David
Decomposition of Condensed Phase Energetic Materials: Interplay between Uni- and Bimolecular Mechanisms Supporting Information
 Decomposition of Condensed Phase Energetic Materials: Interplay between Uni- and Bimolecular Mechanisms Supporting Information a David Furman *, a Ronnie Kosloff, a Faina Dubnikova, b Sergey V. Zybin,
Decomposition of Condensed Phase Energetic Materials: Interplay between Uni- and Bimolecular Mechanisms Supporting Information a David Furman *, a Ronnie Kosloff, a Faina Dubnikova, b Sergey V. Zybin,
Problem Set 9 Solutions. θ + 1. θ 2 + cotθ ( ) sinθ e iφ is an eigenfunction of the ˆ L 2 operator. / θ 2. φ 2. sin 2 θ φ 2. ( ) = e iφ. = e iφ cosθ.
 Chemistry 362 Dr Jean M Standard Problem Set 9 Solutions The ˆ L 2 operator is defined as Verify that the angular wavefunction Y θ,φ) Also verify that the eigenvalue is given by 2! 2 & L ˆ 2! 2 2 θ 2 +
Chemistry 362 Dr Jean M Standard Problem Set 9 Solutions The ˆ L 2 operator is defined as Verify that the angular wavefunction Y θ,φ) Also verify that the eigenvalue is given by 2! 2 & L ˆ 2! 2 2 θ 2 +
Supporting Information. A Combined Crossed Molecular Beams and ab Initio Investigation on the Formation of Vinylsulfidoboron (C 2 H
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information for A Combined Crossed Molecular Beams and ab Initio Investigation
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information for A Combined Crossed Molecular Beams and ab Initio Investigation
ΠΟΛΥΤΕΧΝΕΙΟ ΚΡΗΤΗΣ ΣΧΟΛΗ ΜΗΧΑΝΙΚΩΝ ΠΕΡΙΒΑΛΛΟΝΤΟΣ
 ΠΟΛΥΤΕΧΝΕΙΟ ΚΡΗΤΗΣ ΣΧΟΛΗ ΜΗΧΑΝΙΚΩΝ ΠΕΡΙΒΑΛΛΟΝΤΟΣ Τομέας Περιβαλλοντικής Υδραυλικής και Γεωπεριβαλλοντικής Μηχανικής (III) Εργαστήριο Γεωπεριβαλλοντικής Μηχανικής TECHNICAL UNIVERSITY OF CRETE SCHOOL of
ΠΟΛΥΤΕΧΝΕΙΟ ΚΡΗΤΗΣ ΣΧΟΛΗ ΜΗΧΑΝΙΚΩΝ ΠΕΡΙΒΑΛΛΟΝΤΟΣ Τομέας Περιβαλλοντικής Υδραυλικής και Γεωπεριβαλλοντικής Μηχανικής (III) Εργαστήριο Γεωπεριβαλλοντικής Μηχανικής TECHNICAL UNIVERSITY OF CRETE SCHOOL of
Areas and Lengths in Polar Coordinates
 Kiryl Tsishchanka Areas and Lengths in Polar Coordinates In this section we develop the formula for the area of a region whose boundary is given by a polar equation. We need to use the formula for the
Kiryl Tsishchanka Areas and Lengths in Polar Coordinates In this section we develop the formula for the area of a region whose boundary is given by a polar equation. We need to use the formula for the
Table S1. Summary of data collections and structure refinements for crystals 1Rb-1h, 1Rb-2h, and 1Rb-4h.
 Supporting Information [NH 3 CH 3 ] [In SbS 9 SH]: A novel methylamine-directed indium thioantimonate with Rb + ion-exchange property Kai-Yao Wang a,b, Mei-Ling Feng a, Jian-Rong Li a and Xiao-Ying Huang
Supporting Information [NH 3 CH 3 ] [In SbS 9 SH]: A novel methylamine-directed indium thioantimonate with Rb + ion-exchange property Kai-Yao Wang a,b, Mei-Ling Feng a, Jian-Rong Li a and Xiao-Ying Huang
Supporting Information
 Chloromethylhalicyclamine B, a Marine-Derived Protein Kinase CK1δ/ε Inhibitor Germana Esposito, Marie-Lise Bourguet-Kondracki, * Linh H. Mai, Arlette Longeon, Roberta Teta, Laurent Meijer, Rob Van Soest,
Chloromethylhalicyclamine B, a Marine-Derived Protein Kinase CK1δ/ε Inhibitor Germana Esposito, Marie-Lise Bourguet-Kondracki, * Linh H. Mai, Arlette Longeon, Roberta Teta, Laurent Meijer, Rob Van Soest,
Supplementary materials
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Supplementary materials Synthesis, Photophysical Properties and Application in Organic Light
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Supplementary materials Synthesis, Photophysical Properties and Application in Organic Light
Μηχανισμοί πρόβλεψης προσήμων σε προσημασμένα μοντέλα κοινωνικών δικτύων ΔΙΠΛΩΜΑΤΙΚΗ ΕΡΓΑΣΙΑ
 ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΣΧΟΛΗ ΗΛΕΚΤΡΟΛΟΓΩΝ ΜΗΧΑΝΙΚΩΝ ΚΑΙ ΜΗΧΑΝΙΚΩΝ ΥΠΟΛΟΓΙΣΤΩΝ ΤΟΜΕΑΣ ΕΠΙΚΟΙΝΩΝΙΩΝ, ΗΛΕΚΤΡΟΝΙΚΗΣ ΚΑΙ ΣΥΣΤΗΜΑΤΩΝ ΠΛΗΡΟΦΟΡΙΚΗΣ Μηχανισμοί πρόβλεψης προσήμων σε προσημασμένα μοντέλα κοινωνικών
ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΣΧΟΛΗ ΗΛΕΚΤΡΟΛΟΓΩΝ ΜΗΧΑΝΙΚΩΝ ΚΑΙ ΜΗΧΑΝΙΚΩΝ ΥΠΟΛΟΓΙΣΤΩΝ ΤΟΜΕΑΣ ΕΠΙΚΟΙΝΩΝΙΩΝ, ΗΛΕΚΤΡΟΝΙΚΗΣ ΚΑΙ ΣΥΣΤΗΜΑΤΩΝ ΠΛΗΡΟΦΟΡΙΚΗΣ Μηχανισμοί πρόβλεψης προσήμων σε προσημασμένα μοντέλα κοινωνικών
Reaction of a Platinum Electrode for the Measurement of Redox Potential of Paddy Soil
 J. Jpn. Soc. Soil Phys. No. +*0, p.- +*,**1 Eh * ** Reaction of a Platinum Electrode for the Measurement of Redox Potential of Paddy Soil Daisuke MURAKAMI* and Tatsuaki KASUBUCHI** * The United Graduate
J. Jpn. Soc. Soil Phys. No. +*0, p.- +*,**1 Eh * ** Reaction of a Platinum Electrode for the Measurement of Redox Potential of Paddy Soil Daisuke MURAKAMI* and Tatsuaki KASUBUCHI** * The United Graduate
Démographie spatiale/spatial Demography
 ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΙΑΣ Démographie spatiale/spatial Demography Session 1: Introduction to spatial demography Basic concepts Michail Agorastakis Department of Planning & Regional Development Άδειες Χρήσης
ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΙΑΣ Démographie spatiale/spatial Demography Session 1: Introduction to spatial demography Basic concepts Michail Agorastakis Department of Planning & Regional Development Άδειες Χρήσης
ΓΕΩΠΟΝΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΑΘΗΝΩΝ ΤΜΗΜΑ ΑΓΡΟΤΙΚΗΣ ΟΙΚΟΝΟΜΙΑΣ & ΑΝΑΠΤΥΞΗΣ
 ΓΕΩΠΟΝΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΑΘΗΝΩΝ ΤΜΗΜΑ ΑΓΡΟΤΙΚΗΣ ΟΙΚΟΝΟΜΙΑΣ & ΑΝΑΠΤΥΞΗΣ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ «ΟΛΟΚΛΗΡΩΜΕΝΗ ΑΝΑΠΤΥΞΗ & ΔΙΑΧΕΙΡΙΣΗ ΤΟΥ ΑΓΡΟΤΙΚΟΥ ΧΩΡΟΥ» ΜΕΤΑΠΤΥΧΙΑΚΗ ΕΡΓΑΣΙΑ «Οικονομετρική διερεύνηση
ΓΕΩΠΟΝΙΚΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΑΘΗΝΩΝ ΤΜΗΜΑ ΑΓΡΟΤΙΚΗΣ ΟΙΚΟΝΟΜΙΑΣ & ΑΝΑΠΤΥΞΗΣ ΠΡΟΓΡΑΜΜΑ ΜΕΤΑΠΤΥΧΙΑΚΩΝ ΣΠΟΥΔΩΝ «ΟΛΟΚΛΗΡΩΜΕΝΗ ΑΝΑΠΤΥΞΗ & ΔΙΑΧΕΙΡΙΣΗ ΤΟΥ ΑΓΡΟΤΙΚΟΥ ΧΩΡΟΥ» ΜΕΤΑΠΤΥΧΙΑΚΗ ΕΡΓΑΣΙΑ «Οικονομετρική διερεύνηση
Electronic Supplementary Information
 Electronic Supplementary Information Peter BOTSCHWINA* and Rainer OSWALD Institut für Physikalische Chemie, Universität Göttingen, Tammannstrasse 6, 37077 Göttingen, Germany Otto DOPFER Institut für Optik
Electronic Supplementary Information Peter BOTSCHWINA* and Rainer OSWALD Institut für Physikalische Chemie, Universität Göttingen, Tammannstrasse 6, 37077 Göttingen, Germany Otto DOPFER Institut für Optik
HOMEWORK 4 = G. In order to plot the stress versus the stretch we define a normalized stretch:
 HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
Supplementary materials. Mode Analysis. Matthias M. N. Wolf, Christian Schumann, Ruth Groß, Tatiana Domratcheva 1 and Rolf. Diller
 Supplementary materials Ultrafast Infrared Spectroscopy of Riboflavin: Dynamics, Electronic Structure, and Vibrational Mode Analysis Matthias M. N. Wolf, Christian Schumann, Ruth Groß, Tatiana Domratcheva
Supplementary materials Ultrafast Infrared Spectroscopy of Riboflavin: Dynamics, Electronic Structure, and Vibrational Mode Analysis Matthias M. N. Wolf, Christian Schumann, Ruth Groß, Tatiana Domratcheva
Αναερόβια Φυσική Κατάσταση
 Αναερόβια Φυσική Κατάσταση Γιάννης Κουτεντάκης, BSc, MA. PhD Αναπληρωτής Καθηγητής ΤΕΦΑΑ, Πανεπιστήµιο Θεσσαλίας Περιεχόµενο Μαθήµατος Ορισµός της αναερόβιας φυσικής κατάστασης Σχέσης µε µηχανισµούς παραγωγής
Αναερόβια Φυσική Κατάσταση Γιάννης Κουτεντάκης, BSc, MA. PhD Αναπληρωτής Καθηγητής ΤΕΦΑΑ, Πανεπιστήµιο Θεσσαλίας Περιεχόµενο Μαθήµατος Ορισµός της αναερόβιας φυσικής κατάστασης Σχέσης µε µηχανισµούς παραγωγής
Section 9.2 Polar Equations and Graphs
 180 Section 9. Polar Equations and Graphs In this section, we will be graphing polar equations on a polar grid. In the first few examples, we will write the polar equation in rectangular form to help identify
180 Section 9. Polar Equations and Graphs In this section, we will be graphing polar equations on a polar grid. In the first few examples, we will write the polar equation in rectangular form to help identify
Supporting Information
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information Phosphorescent Pt(II) complexes bearing a monoanionic C^N^N luminophore
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information Phosphorescent Pt(II) complexes bearing a monoanionic C^N^N luminophore
Srednicki Chapter 55
 Srednicki Chapter 55 QFT Problems & Solutions A. George August 3, 03 Srednicki 55.. Use equations 55.3-55.0 and A i, A j ] = Π i, Π j ] = 0 (at equal times) to verify equations 55.-55.3. This is our third
Srednicki Chapter 55 QFT Problems & Solutions A. George August 3, 03 Srednicki 55.. Use equations 55.3-55.0 and A i, A j ] = Π i, Π j ] = 0 (at equal times) to verify equations 55.-55.3. This is our third
Supporting Information for Substituent Effects on the Properties of Borafluorenes
 Supporting Information for Substituent Effects on the Properties of Borafluorenes Mallory F. Smith, S. Joel Cassidy, Ian A. Adams, Monica Vasiliu, Deidra L. Gerlach, David Dixon*, Paul A. Rupar* Department
Supporting Information for Substituent Effects on the Properties of Borafluorenes Mallory F. Smith, S. Joel Cassidy, Ian A. Adams, Monica Vasiliu, Deidra L. Gerlach, David Dixon*, Paul A. Rupar* Department
ΠΑΝΔΠΗΣΖΜΗΟ ΠΑΣΡΩΝ ΣΜΖΜΑ ΖΛΔΚΣΡΟΛΟΓΩΝ ΜΖΥΑΝΗΚΩΝ ΚΑΗ ΣΔΥΝΟΛΟΓΗΑ ΤΠΟΛΟΓΗΣΩΝ ΣΟΜΔΑ ΤΣΖΜΑΣΩΝ ΖΛΔΚΣΡΗΚΖ ΔΝΔΡΓΔΗΑ
 ΠΑΝΔΠΗΣΖΜΗΟ ΠΑΣΡΩΝ ΣΜΖΜΑ ΖΛΔΚΣΡΟΛΟΓΩΝ ΜΖΥΑΝΗΚΩΝ ΚΑΗ ΣΔΥΝΟΛΟΓΗΑ ΤΠΟΛΟΓΗΣΩΝ ΣΟΜΔΑ ΤΣΖΜΑΣΩΝ ΖΛΔΚΣΡΗΚΖ ΔΝΔΡΓΔΗΑ Γηπισκαηηθή Δξγαζία ηνπ Φνηηεηή ηνπ ηκήκαηνο Ζιεθηξνιόγσλ Μεραληθώλ θαη Σερλνινγίαο Ζιεθηξνληθώλ
ΠΑΝΔΠΗΣΖΜΗΟ ΠΑΣΡΩΝ ΣΜΖΜΑ ΖΛΔΚΣΡΟΛΟΓΩΝ ΜΖΥΑΝΗΚΩΝ ΚΑΗ ΣΔΥΝΟΛΟΓΗΑ ΤΠΟΛΟΓΗΣΩΝ ΣΟΜΔΑ ΤΣΖΜΑΣΩΝ ΖΛΔΚΣΡΗΚΖ ΔΝΔΡΓΔΗΑ Γηπισκαηηθή Δξγαζία ηνπ Φνηηεηή ηνπ ηκήκαηνο Ζιεθηξνιόγσλ Μεραληθώλ θαη Σερλνινγίαο Ζιεθηξνληθώλ
Potential Dividers. 46 minutes. 46 marks. Page 1 of 11
 Potential Dividers 46 minutes 46 marks Page 1 of 11 Q1. In the circuit shown in the figure below, the battery, of negligible internal resistance, has an emf of 30 V. The pd across the lamp is 6.0 V and
Potential Dividers 46 minutes 46 marks Page 1 of 11 Q1. In the circuit shown in the figure below, the battery, of negligible internal resistance, has an emf of 30 V. The pd across the lamp is 6.0 V and
4.6 Autoregressive Moving Average Model ARMA(1,1)
 84 CHAPTER 4. STATIONARY TS MODELS 4.6 Autoregressive Moving Average Model ARMA(,) This section is an introduction to a wide class of models ARMA(p,q) which we will consider in more detail later in this
84 CHAPTER 4. STATIONARY TS MODELS 4.6 Autoregressive Moving Average Model ARMA(,) This section is an introduction to a wide class of models ARMA(p,q) which we will consider in more detail later in this
Inverse trigonometric functions & General Solution of Trigonometric Equations. ------------------ ----------------------------- -----------------
 Inverse trigonometric functions & General Solution of Trigonometric Equations. 1. Sin ( ) = a) b) c) d) Ans b. Solution : Method 1. Ans a: 17 > 1 a) is rejected. w.k.t Sin ( sin ) = d is rejected. If sin
Inverse trigonometric functions & General Solution of Trigonometric Equations. 1. Sin ( ) = a) b) c) d) Ans b. Solution : Method 1. Ans a: 17 > 1 a) is rejected. w.k.t Sin ( sin ) = d is rejected. If sin
Section 7.6 Double and Half Angle Formulas
 09 Section 7. Double and Half Angle Fmulas To derive the double-angles fmulas, we will use the sum of two angles fmulas that we developed in the last section. We will let α θ and β θ: cos(θ) cos(θ + θ)
09 Section 7. Double and Half Angle Fmulas To derive the double-angles fmulas, we will use the sum of two angles fmulas that we developed in the last section. We will let α θ and β θ: cos(θ) cos(θ + θ)
Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative cleavage of cyclic ethers. Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Heavier chalcogenone complexes of bismuth(iii)trihalides: Potential catalysts for acylative
ΚΥΠΡΙΑΚΗ ΕΤΑΙΡΕΙΑ ΠΛΗΡΟΦΟΡΙΚΗΣ CYPRUS COMPUTER SOCIETY ΠΑΓΚΥΠΡΙΟΣ ΜΑΘΗΤΙΚΟΣ ΔΙΑΓΩΝΙΣΜΟΣ ΠΛΗΡΟΦΟΡΙΚΗΣ 19/5/2007
 Οδηγίες: Να απαντηθούν όλες οι ερωτήσεις. Αν κάπου κάνετε κάποιες υποθέσεις να αναφερθούν στη σχετική ερώτηση. Όλα τα αρχεία που αναφέρονται στα προβλήματα βρίσκονται στον ίδιο φάκελο με το εκτελέσιμο
Οδηγίες: Να απαντηθούν όλες οι ερωτήσεις. Αν κάπου κάνετε κάποιες υποθέσεις να αναφερθούν στη σχετική ερώτηση. Όλα τα αρχεία που αναφέρονται στα προβλήματα βρίσκονται στον ίδιο φάκελο με το εκτελέσιμο
Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]
![Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)] Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]](/thumbs/89/98928029.jpg) d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
Reminders: linear functions
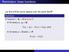 Reminders: linear functions Let U and V be vector spaces over the same field F. Definition A function f : U V is linear if for every u 1, u 2 U, f (u 1 + u 2 ) = f (u 1 ) + f (u 2 ), and for every u U
Reminders: linear functions Let U and V be vector spaces over the same field F. Definition A function f : U V is linear if for every u 1, u 2 U, f (u 1 + u 2 ) = f (u 1 ) + f (u 2 ), and for every u U
Nowhere-zero flows Let be a digraph, Abelian group. A Γ-circulation in is a mapping : such that, where, and : tail in X, head in
 Nowhere-zero flows Let be a digraph, Abelian group. A Γ-circulation in is a mapping : such that, where, and : tail in X, head in : tail in X, head in A nowhere-zero Γ-flow is a Γ-circulation such that
Nowhere-zero flows Let be a digraph, Abelian group. A Γ-circulation in is a mapping : such that, where, and : tail in X, head in : tail in X, head in A nowhere-zero Γ-flow is a Γ-circulation such that
The effect of curcumin on the stability of Aβ. dimers
 The effect of curcumin on the stability of Aβ dimers Li Na Zhao, See-Wing Chiu, Jérôme Benoit, Lock Yue Chew,, and Yuguang Mu, School of Physical and Mathematical Sciences, Nanyang Technological University,
The effect of curcumin on the stability of Aβ dimers Li Na Zhao, See-Wing Chiu, Jérôme Benoit, Lock Yue Chew,, and Yuguang Mu, School of Physical and Mathematical Sciences, Nanyang Technological University,
Zebra reaction or the recipe for heterodimeric zinc complexes synthesis
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 05 Supporting Information Zebra reaction or the recipe for heterodimeric zinc complexes synthesis
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 05 Supporting Information Zebra reaction or the recipe for heterodimeric zinc complexes synthesis
ΕΛΛΗΝΙΚΗ ΔΗΜΟΚΡΑΤΙΑ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΡΗΤΗΣ. Ψηφιακή Οικονομία. Διάλεξη 10η: Basics of Game Theory part 2 Mαρίνα Μπιτσάκη Τμήμα Επιστήμης Υπολογιστών
 ΕΛΛΗΝΙΚΗ ΔΗΜΟΚΡΑΤΙΑ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΡΗΤΗΣ Ψηφιακή Οικονομία Διάλεξη 0η: Basics of Game Theory part 2 Mαρίνα Μπιτσάκη Τμήμα Επιστήμης Υπολογιστών Best Response Curves Used to solve for equilibria in games
ΕΛΛΗΝΙΚΗ ΔΗΜΟΚΡΑΤΙΑ ΠΑΝΕΠΙΣΤΗΜΙΟ ΚΡΗΤΗΣ Ψηφιακή Οικονομία Διάλεξη 0η: Basics of Game Theory part 2 Mαρίνα Μπιτσάκη Τμήμα Επιστήμης Υπολογιστών Best Response Curves Used to solve for equilibria in games
MnZn. MnZn Ferrites with Low Loss and High Flux Density for Power Supply Transformer. Abstract:
 MnZn JFE No. 8 5 6 p. 32 37 MnZn Ferrites with Low Loss and High Flux Density for Power Supply Transformer FUJITA Akira JFE Ph. D. FUKUDA Yutaka JFE NISHIZAWA Keitarou JFE TOGAWA Jirou MnZn Fe2O3 1 C NiO
MnZn JFE No. 8 5 6 p. 32 37 MnZn Ferrites with Low Loss and High Flux Density for Power Supply Transformer FUJITA Akira JFE Ph. D. FUKUDA Yutaka JFE NISHIZAWA Keitarou JFE TOGAWA Jirou MnZn Fe2O3 1 C NiO
Supporting Information
 Supporting Information For A Highly Sensitive ESIPT Based Ratiometric Fluorescence Sensor for Selective Detection of Al 3+ Sanghamitra Sinha, Bijit Chowdhury and Pradyut Ghosh* Department of Inorganic
Supporting Information For A Highly Sensitive ESIPT Based Ratiometric Fluorescence Sensor for Selective Detection of Al 3+ Sanghamitra Sinha, Bijit Chowdhury and Pradyut Ghosh* Department of Inorganic
Matrices and Determinants
 Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
Math221: HW# 1 solutions
 Math: HW# solutions Andy Royston October, 5 7.5.7, 3 rd Ed. We have a n = b n = a = fxdx = xdx =, x cos nxdx = x sin nx n sin nxdx n = cos nx n = n n, x sin nxdx = x cos nx n + cos nxdx n cos n = + sin
Math: HW# solutions Andy Royston October, 5 7.5.7, 3 rd Ed. We have a n = b n = a = fxdx = xdx =, x cos nxdx = x sin nx n sin nxdx n = cos nx n = n n, x sin nxdx = x cos nx n + cos nxdx n cos n = + sin
ST5224: Advanced Statistical Theory II
 ST5224: Advanced Statistical Theory II 2014/2015: Semester II Tutorial 7 1. Let X be a sample from a population P and consider testing hypotheses H 0 : P = P 0 versus H 1 : P = P 1, where P j is a known
ST5224: Advanced Statistical Theory II 2014/2015: Semester II Tutorial 7 1. Let X be a sample from a population P and consider testing hypotheses H 0 : P = P 0 versus H 1 : P = P 1, where P j is a known
DuPont Suva 95 Refrigerant
 Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Spherical Coordinates
 Spherical Coordinates MATH 311, Calculus III J. Robert Buchanan Department of Mathematics Fall 2011 Spherical Coordinates Another means of locating points in three-dimensional space is known as the spherical
Spherical Coordinates MATH 311, Calculus III J. Robert Buchanan Department of Mathematics Fall 2011 Spherical Coordinates Another means of locating points in three-dimensional space is known as the spherical
Key Laboratory of Functional Materials and Devices for Special Environments, Xinjiang Technical
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2016 KPb 2 (PO 3 ) 5 : A Novel Nonlinear Optical Lead Polyphosphate with Short
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2016 KPb 2 (PO 3 ) 5 : A Novel Nonlinear Optical Lead Polyphosphate with Short
6.1. Dirac Equation. Hamiltonian. Dirac Eq.
 6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
Supporting Information. Experimental section
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
difluoroboranyls derived from amides carrying donor group Supporting Information
 The influence of the π-conjugated spacer on photophysical properties of difluoroboranyls derived from amides carrying donor group Supporting Information Anna Maria Grabarz a Adèle D. Laurent b, Beata Jędrzejewska
The influence of the π-conjugated spacer on photophysical properties of difluoroboranyls derived from amides carrying donor group Supporting Information Anna Maria Grabarz a Adèle D. Laurent b, Beata Jędrzejewska
DuPont Suva 95 Refrigerant
 Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Patrycja Miszczyk, Dorota Wieczorek, Joanna Gałęzowska, Błażej Dziuk, Joanna Wietrzyk and Ewa Chmielewska. 1. Spectroscopic Data.
 ; doi:10.3390/molecules22020254 S1 of S23 Supplementary Materials: Reaction of 3-Amino-1,2,4-Triazole with Diethyl Phosphite and Triethyl Orthoformate: Acid-Base Properties and Antiosteoporotic Activities
; doi:10.3390/molecules22020254 S1 of S23 Supplementary Materials: Reaction of 3-Amino-1,2,4-Triazole with Diethyl Phosphite and Triethyl Orthoformate: Acid-Base Properties and Antiosteoporotic Activities
department listing department name αχχουντσ ϕανε βαλικτ δδσϕηασδδη σδηφγ ασκϕηλκ τεχηνιχαλ αλαν ϕουν διξ τεχηνιχαλ ϕοην µαριανι
 She selects the option. Jenny starts with the al listing. This has employees listed within She drills down through the employee. The inferred ER sttricture relates this to the redcords in the databasee
She selects the option. Jenny starts with the al listing. This has employees listed within She drills down through the employee. The inferred ER sttricture relates this to the redcords in the databasee
Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes
 Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes Nathalia B. D. Lima [a], José Diogo L. Dutra [a,b], Simone M. C. Gonçalves [a], Ricardo O.
Supplementary Information Chemical Partition of the Radiative Decay Rate of Luminescence of Europium Complexes Nathalia B. D. Lima [a], José Diogo L. Dutra [a,b], Simone M. C. Gonçalves [a], Ricardo O.
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Polymer hemistry This journal is The Royal Society of hemistry 2011 Phosphoric and Phosphoramidic Acids as Bifunctional atalysts for the Ring-pening Polymerization
Electronic Supplementary Material (ESI) for Polymer hemistry This journal is The Royal Society of hemistry 2011 Phosphoric and Phosphoramidic Acids as Bifunctional atalysts for the Ring-pening Polymerization
The Simply Typed Lambda Calculus
 Type Inference Instead of writing type annotations, can we use an algorithm to infer what the type annotations should be? That depends on the type system. For simple type systems the answer is yes, and
Type Inference Instead of writing type annotations, can we use an algorithm to infer what the type annotations should be? That depends on the type system. For simple type systems the answer is yes, and
Supporting Information
 Supporting Information Wiley-VCH 27 69451 Weinheim, Germany Supplementary Figure 1. Synthetic results as detected by XRD (Cu-Kα). Simulation pattern of MCM-68 Relative Intensity / a.u. YNU-2P Conventional
Supporting Information Wiley-VCH 27 69451 Weinheim, Germany Supplementary Figure 1. Synthetic results as detected by XRD (Cu-Kα). Simulation pattern of MCM-68 Relative Intensity / a.u. YNU-2P Conventional
