Malgorzata Korycka-Machala, Marcin Nowosielski, Aneta Kuron, Sebastian Rykowski, Agnieszka Olejniczak, Marcin Hoffmann and Jaroslaw Dziadek
|
|
|
- Σόλων Κοτζιάς
- 5 χρόνια πριν
- Προβολές:
Transcript
1 Molecules 2017, 21, 154; doi: /molecules Supplementary Materials: Naphthalimides Selectively Inhibit the Activity of Bacterial, Replicative DNA Ligases and Display Bactericidal Effect against Tubercle Bacilli Malgorzata Korycka-Machala, Marcin Nowosielski, Aneta Kuron, Sebastian Rykowski, Agnieszka Olejniczak, Marcin Hoffmann and Jaroslaw Dziadek S1 of S19 Figure S1. Efficiency of a double-stranded DNA 40-bp ligation by M. tuberculosis LigA. SNaP-Shot analysis on Genetic Analyzer 3500.
2 S2 of S19 Figure S2. DNA (40-bp) ligation efficiency as a function of the M. tuberculosis ligase A concentration. Figure S3. DNA (40-bp) ligation efficiency as a function of time (M. tuberculosis, 8.7 ng/µl). Figure S4. DNA (40-bp) ligation efficiency at different temperatures (M. tuberculosis, 8.7 ng/µl).
3 S3 of S19 Figure S5. ESI spectrum of pinafide. ESI-MS: m/z 340 [M + 1] +, calcd. for C18H17N3O4: Figure S6. 1 H-NMR spectrum of pinafide. 1 H-NMR (DMSO): δ (ppm) = 9.46 (d, 1H, H-4), 8.95 (d, 1H, H-6), 8.77 (dd, 1H, H-9), 8.67 (dd, 1H, H-8), 8.05 (q, 1H, H-7), 4.19 (t, 2H, H-2), 2.70 (t, 2H, H-1), (m, 4H, pyrrolidine, overlapping with DMSO), (m, 4H, pyrrolidine). N O N O NO 2 Figure S7. ESI spectrum of mitonafide. ESI-MS: m/z 314 [M + 1] +, calcd. for C16H15N3O4:
4 S4 of S19 Figure S8. 1 H-NMR spectrum of mitonafide. 1 H-NMR (DMSO): δ (ppm) = 9.74 (d, 1H, H-4), 9.23 (d, 1H, H-6), 9.05 (dd, 1H, H-9), 8.95 (dd, 1H, H-8), 8.33 (t, 1H, H-7), 4.45 (t, 2H, H-2), (m, 8H, H-1, N-CH3, N-CH3). Electronic Supporting Information 1 H-NMR spectrum was recorded on a Bruker Avance 600 MHz spectrometer equipped with a TBI probe. The spectrum for 1 H nuclei was recorded at MHz. For NMR, the following solvent was used: DMSO-d6 (δh = 2.50 ppm). All chemical shifts (δ) are quoted in parts per million (ppm). The following abbreviations were used to denote the multiplicities: d = doublet, dd = doublet of doublets, t = triplet, q = quartet, and m = multiplet. Mass spectra were recorded on a CombiFlash PurIon Model Eurus35 (Teledyne ISCO, Lincoln, NE, USA). The ionization was achieved by electrospray ionization in the positive ion mode (ESI+) and negative ion mode (ESI ). The capillary voltage was set to 3.5 kv. The source temperature was 200 C, and the desolvation temperature was 200 C. Nitrogen was used as a desolvation gas (flow 35 L/min, purity >99%). The theoretical molecular masses of the compounds were calculated using the Show Analysis Window option in the ChemDraw Ultra 12.0 program. The calculated m/z corresponds to the average mass of the compounds consisting of natural isotopes. Table S1. In vitro inhibition of DNA LigA by compound NSC Conc. (µm) E. coli Ligation (%) M. tuberculosis T4 0 (control)
5 Table S2. In vitro inhibition of DNA LigA by compound NSC S5 of S19 Conc. (µm) E. coli Ligation (%) M. tuberculosis T4 0 (control) Table S3. In vitro inhibition of DNA LigA by compound NSC5856. Conc. (µm) E. coli Ligation (%) M. tuberculosis T4 0 (control) Table S4. In vitro inhibition of DNA LigA by compound NSC Conc. (µm) E. coli Ligation (%) M. tuberculosis T4 0 (control) Table S5. In vitro inhibition of DNA LigA by compound NSC Conc. (µm) E. coli Ligation (%) M. tuberculosis T4 0 (control) Table S6. In vitro inhibition of DNA LigA by compound NSC Conc. (µm) Ligation (%) E. coli M. tuberculosis T4 0 (control)
6 Table S7. In vitro inhibition of DNA LigA by compound NSC S6 of S19 Conc. (µm) E. coli Ligation (%) M. tuberculosis T4 0 (control) Table S8. In vitro inhibition of DNA LigA by compound NSC Conc. (µm) E. coli Ligation (%) M. tuberculosis T4 0 (control) Table S9. In vitro inhibition of DNA LigA by mitonafide. Conc. (µm) E. coli Ligation (%) M. tuberculosis T4 0 (control) Table S10. Results of docking to the open enzyme structure (PDB: 1ZAU). Lp. NCI ZINC Docking ( Open ) (K2) (M2) (M2) (V1) (N1) (N1) (Z1) (G3) (G3) (Q1) (Q1) O2) (O2) Table S dock. Amino Acid Etotal (kj/mol) EAA (kj/mol) Eli g (kj/mol) Eint (kj/mol) LYS 123 6,499, ,107, ,391, ALA 128 6,043, , ,391, ALA 322 6,043, , ,391, ARG 182 6,786, ,395, ,391, ARG 211 6,786, ,395, ,391, ASN 209 6,486, ,094, ,391, ASP 125 6,537, ,145, ,391, ASP 291 6,537, ,145, ,391, ASP 295 6,537, ,145, ,391, CYS 235 7,089, ,697, ,391,
7 GLU 121 6,640, ,248, ,391, GLU 184 6,640, ,248, ,391, GLU 265 6,625, ,245, ,380, GLU 293 6,640, ,248, ,391, GLY 126 5,940, , ,391, GLY 183 5,940, , ,391, GLY 296 5,940, , ,391, HSD 236 6,634, ,242, ,391, HSD 266 6,634, ,242, ,391, HSD 292 6,634, ,242, ,391, ILE 124 6,352, , ,391, ILE 234 6,353, , ,391, ILE 294 6,353, , ,391, S7 of S19 Table S min. Amino Acid Etotal (kj/mol) EAA (kj/mol) Eli g (kj/mol) Eint (kj/mol) ALA 128 6,044, , ,392, ALA 322 6,044, , ,392, ARG 144 6,787, ,395, ,392, ARG 182 6,771, ,391, ,380, ARG 211 6,787, ,395, ,392, ASN 209 6,486, ,094, ,392, ASP 125 6,537, ,145, ,392, ASP 291 6,537, ,145, ,392, ASP 295 6,537, ,145, ,392, CYS 235 7,089, ,697, ,392, GLU 121 6,640, ,248, ,392, GLU 184 6,640, ,248, ,392, GLU 265 6,640, ,248, ,392, GLU 293 6,640, ,248, ,392, GLY 126 5,940, , ,392, GLY 183 5,940, , ,392, HSD 236 6,634, ,242, ,392, HSD 266 6,634, ,242, ,392, HSD 292 6,634, ,242, ,392, ILE 124 6,353, , ,392, ILE 234 6,353, , ,392, ILE 294 6,353, , ,392, ILE 321 6,353, , ,392, Table S dock. Amino Acid Etotal (kj/mol) EAA (kj/mol) Eli g (kj/mol) Eint (kj/mol) ASP 93 5,159, ,145, ,013, HSD 292 5,256, ,242, ,013, LYS 324 5,121, ,107, ,013, ALA 128 4,665, , ,013, ALA 322 4,665, , ,013, ARG 144 5,408, ,395, ,013, ARG 182 5,408, ,395, ,013, ASN 94 5,108, ,094, ,013, ASP 125 5,159, ,145, ,013, ASP 291 5,159, ,145, ,013, ILE 234 4,975, , ,013,
8 ILE 294 4,975, , ,013, LEU 122 4,975, , ,013, LEU 90 4,975, , ,013, LEU 92 4,975, , ,013, LYS 123 5,121, ,107, ,013, LYS 300 5,121, ,107, ,013, MET 89 5,917, ,903, ,013, SER 264 4,863, , ,013, SER 91 4,863, , ,013, TYR 253 5,469, ,455, ,013, VAL 263 4,872, , ,013, VAL 290 4,871, , ,013, S8 of S19 Table S14. 4,783,229 min. Amino Acid Etotal (kj/mol) EAA (kj/mol) Eli g (kj/mol) Eint (kj/mo) GLU 184 5,262, ,248, ,014, ALA 128 4,666, , ,014, ALA 322 4,666, , ,014, ARG 144 5,409, ,395, ,014, ARG 182 5,409, ,395, ,014, ASN 94 5,108, ,094, ,014, ASP 125 5,159, ,145, ,014, ASP 291 5,159, ,145, ,014, ASP 93 5,159, ,145, ,014, CYS 235 5,711, ,697, ,014, GLU 121 5,262, ,248, ,014, LEU 122 4,975, , ,014, LEU 90 4,975, , ,014, LEU 92 4,975, , ,014, LYS 123 5,121, ,107, ,014, LYS 300 5,121, ,107, ,014, MET 89 5,917, ,903, ,014, PRO 262 4,869, , ,014, SER 264 4,863, , ,014, SER 91 4,863, , ,014, TYR 253 5,469, ,455, ,014, VAL 263 4,872, , ,014, VAL 290 4,872, , ,014, Table S dock Amino Acid Etotal (kj/mo)] EAA (kj/mol) Eli g (kj/mol) Eint (kj/mol) GLY 239 4,768, , ,219, ALA 247 4,871, , ,219, ALA 252 4,871, , ,219, ALA 316 4,871, , ,219, ARG 182 5,614, ,395, ,219, ARG 245 5,614, ,395, ,219, ARG 308 5,614, ,395, ,219, ARG 309 5,614, ,395, ,219, ARG 318 5,614, ,395, ,219, ASP 302 5,364, ,145, ,219, GLN 251 5,417, ,197, ,219, GLN 307 5,417, ,197, ,219, GLU 180 5,468, ,248, ,219, GLU 242 5,468, ,248, ,219, GLU 87 5,468, ,248, ,219,
9 GLY 237 4,768, , ,219, GLY 243 4,768, , ,219, GLY 311 4,768, , ,219, HSD 240 5,462, ,242, ,219, HSD 250 5,462, ,242, ,219, LEU 238 5,181, , ,219, LEU 249 5,180, , ,219, LEU 310 5,181, , ,219, S9 of S19 Table S min. Amino Acid Etotal (kj/mol) EAA (kj/mol) Eli g (kj/mol) Eint (kj/mol) ARG 88 5,615, ,395, ,219, ALA 252 4,871, , ,219, ALA 316 4,871, , ,219, ARG 182 5,615, ,395, ,219, ARG 245 5,615, ,395, ,219, ARG 308 5,615, ,395, ,219, ARG 318 5,615, ,395, ,219, LEU 238 5,181, , ,219, LEU 249 5,181, , ,219, LEU 310 5,181, , ,219, LEU 86 5,181, , ,219, LEU 90 5,181, , ,219, LYS 300 5,327, ,107, ,219, PHE 244 5,478, ,258, ,219, PRO 246 5,075, , ,219, PRO 317 5,075, , ,219, SER 312 5,069, , ,219, THR 248 5,172, , ,219, THR 313 5,172, , ,219, TRP 319 5,823, ,603, ,219, TYR 253 5,675, ,455, ,219, VAL 241 5,078, , ,219, VAL 304 5,078, , ,219, Table S dock. Amino Acid Etotal (kj/mol) EAA (kj/mol) Eli g (kj/mol) Eint (kj/mol) GLN 307 5,417, ,197, ,219, LEU 238 5,180, , ,219, ALA 252 4,871, , ,219, ALA 305 4,871, , ,219, ALA 320 4,871, , ,219, ARG 182 5,614, ,395, ,219, ARG 245 5,614, ,395, ,219, ARG 308 5,614, ,395, ,219, ARG 309 5,614, ,395, ,219, ARG 318 5,614, ,395, ,219, ASP 302 5,364, ,145, ,219, LEU 249 5,162, , ,208, v.h. LEU 256 5,180, , ,219, LEU 306 5,180, , ,219, LEU 310 5,181, , ,219, LEU 90 5,180, , ,219, LYS 300 5,327, ,107, ,219, PHE 244 5,477, ,258, ,219, PRO 246 5,074, , ,219, PRO 317 5,073, , ,219,
10 SER 312 5,068, , ,219, THR 248 5,172, , ,219, TRP 319 5,823, ,603, ,219, S10 of S19 Table S min. ALA 247 4,871, ,52, ,219, ALA 252 4,871, , ,219, ALA 305 4,871, , ,219, ARG 182 5,615, ,395, ,219, ARG 245 5,615, ,395, ,219, ARG 308 5,615, ,395, ,219, ARG 309 5,615, ,395, ,219, ARG 88 5,615, ,395, ,219, GLN 307 5,417, ,197, ,219, GLU 121 5,468, ,248, ,219, LEU 249 5,181, , ,219, LEU 256 5,181, , ,219, LEU 306 5,181, , ,219, LEU 310 5,181, , ,219, LEU 90 5,181, , ,219, LEU 92 5,181, , ,219, LYS 300 5,327, ,107, ,219, MET 89 6,123, ,903, ,219, PRO 246 5,075, , ,219, PRO 317 5,075, , ,219, SER 312 5,069, , ,219, THR 248 5,172, , ,219, TRP 319 5,823, ,603, ,219, Table S dock. GLY 296 4,504, , ,955, ALA 128 4,607, , ,955, ALA 322 4,607, , ,955, ARG 144 5,350, ,395, ,955, ARG 182 5,350, ,395, ,955, ARG 211 5,350, ,395, ,955, ASP 125 5,100, ,145, ,955, ASP 295 5,100, ,145, ,955, GLU 121 5,204, ,248, ,955, GLU 184 5,204, ,248, ,955, GLU 265 5,204, ,248, ,955, GLU 293 5,204, ,248, ,955, GLY 126 4,504, , ,955, GLY 183 4,504, , ,955, GLY 237 4,504, , ,955, HSD 236 5,198, ,242, ,955, HSD 292 5,198, ,242, ,955, ILE 124 4,916, , ,955, ILE 234 4,917, , ,955, ILE 294 4,917, , ,955, LEU 122 4,917, , ,955, LEU 90 4,917, , ,955, LEU 92 4,917, , ,955,
11 Table S min. S11 of S19 GLY 296 4,504, , ,955, ALA 128 4,607, , ,955, ALA 322 4,607, , ,955, ARG 144 5,351, ,395, ,955, ARG 182 5,351, ,395, ,955, ARG 211 5,351, ,395, ,955, ASP 125 5,101, ,145, ,955, ASP 295 5,101, ,145, ,955, CYS 235 5,653, ,697, ,955, GLU 121 5,204, ,248, ,955, GLU 184 5,204, ,248, ,955, GLU 265 5,204, ,248, ,955, GLY 126 4,504, , ,955, GLY 183 4,504, , ,955, GLY 237 4,504, , ,955, HSD 236 5,198, ,242, ,955, ILE 124 4,917, , ,955, ILE 234 4,917, , ,955, LEU 122 4,917, , ,955, LEU 90 4,917, , ,955, LEU 92 4,917, , ,955, LYS 123 5,063, ,107, ,955, LYS 300 5,051, ,104, ,946, Table S dock. LEU 238 4,917, , ,955, ALA 252 4,607, , ,955, ALA 320 4,607, , ,955, ARG 182 5,350, ,395, ,955, ARG 245 5,350, ,395, ,955, ARG 308 5,350, ,395, ,955, ARG 318 5,350, ,395, ,955, GLN 307 5,153, ,197, ,955, GLU 121 5,204, ,248, ,955, LEU 249 4,916, , ,955, LEU 310 4,917, , ,955, LEU 90 4,917, , ,955, LEU 92 4,917, , ,955, LYS 300 5,063, ,107, ,955, PHE 244 5,213, ,258, ,955, PRO 246 4,810, , ,955, PRO 317 4,809, , ,955, SER 312 4,804, , ,955, THR 248 4,908, , ,9555, TRP 319 5,559, ,603, ,955, TYR 253 5,411, ,55, ,955, VAL 241 4,813, , ,955, VAL 298 4,813, , ,955,
12 Table S min. S12 of S19 Amino Acid Etotal (kj/mol) E AA (kj/mol) Eli g (kj/mol) Eint(kJ/mol) ALA 252 4,607, ,52, ,955, ARG 182 5,351, ,395, ,955, ARG 245 5,351, ,395, ,955, ARG 308 5,351, ,395, ,955, GLN 307 5,153, ,197, ,955, GLU 121 5,204, ,248, ,955, GLU 87 5,204, ,248, ,955, GLY 237 4,504, , ,955, GLY 239 4,504, , ,955, GLY 311 4,504, , ,955, HSD 236 5,198, ,242, ,955, HSD 240 5,198, ,242, ,955, LEU 119 4,917, , ,955, LEU 238 4,917, , ,955, LEU 249 4,917, , ,955, LEU 310 4,917, , ,955, LEU 90 4,917, , ,955, LEU 92 4,917, , ,955, LYS 300 5,063, ,107, ,955, PHE 244 5,214, ,258, ,955, PRO 246 4,811, , ,955, PRO 317 4,811, , ,955, SER 312 4,805, , ,955, Table S dock. ALA 247 5,068, , ,416, ALA 252 5,068, , ,416, ARG 182 5,811, ,395, ,416, ARG 245 5,811, ,395, ,416, ARG 308 5,811, ,395, ,416, ASP 302 5,562, ,145, ,416, GLN 251 5,614, ,197, ,416, GLN 307 5,614, ,197, ,416, GLU 121 5,665, ,248, ,416, GLU 265 5,665, ,248, ,416, GLU 87 5,665, ,248, ,416, GLY 237 4,965, ,488, ,416, GLY 239 4,965, ,488, ,416, HSD 236 5,659, ,242, ,416, HSD 240 5,659, ,242, ,416, HSD 250 5,659, ,242, ,416, LEU 119 5,378, , ,416, LEU 238 5,378, , ,416, LEU ,77, , ,416, LEU 90 5,378, , ,416, LYS 300 5,524, ,107, ,416, PHE 244 5,674, ,258, ,416, PRO 246 5,271, , ,416,
13 Table S dock. S13 of S19 ALA 247 5,068, , ,416, ALA 252 5,068, , ,416, ARG 182 5,811, ,395, ,416, ARG 245 5,811, ,395, ,416, ARG 308 5,811, ,395, ,416, ASP 302 5,562, ,145, ,416, GLN 251 5,614, ,197, ,416, GLN 307 5,614, ,197, ,416, GLU 121 5,665, ,248, ,416, GLU 265 5,665, ,248, ,416, GLU 87 5,665, ,248, ,416, GLY 237 4,965, , ,416, GLY 239 4,965, , ,416, HSD 236 5,659, ,242, ,416, HSD 240 5,659, ,242, ,416, HSD 250 5,659, ,242, ,416, LEU 119 5,378, , ,416, LEU 238 5,378, , ,416, LEU 249 5,377, , ,416, LEU 90 5,378, , ,416, LYS 300 5,524, ,107, ,416, PHE 244 5,674, ,258, ,416, PRO 246 5,271, , ,416, Table S min. ALA 252 5,069, , ,416, ARG 182 5,812, ,395, ,416, ARG 245 5,812, ,395, ,416, ARG 308 5,812, ,395, ,416, ASP 302 5,562, ,145, ,416, GLN 307 5,614, ,197, ,416, GLU 121 5,665, ,248, ,416, GLU 87 5,666,046.00,1248, ,416, GLY 237 4,965, , ,416, GLY 239 4,965, , ,416, HSD 236 5,659, ,242, ,416, HSD 240 5,659, ,242, ,416, HSD 250 5,659, ,242, ,416, LEU 238 5,378, , ,416, LEU 249 5,378, , ,416, LEU 90 5,378, , ,416, LEU 92 5,378, , ,416, LYS 300 5,524, ,107, ,416, PHE 244 5,675, ,258, ,416, PRO 246 5,272, , ,416, PRO 317 5,272, , ,416, SER 312 5,266, , ,416, THR 248 5,369, , ,416,
14 Table S dock. S14 of S19 LEU 238 5,378, , ,416, ALA 252 5,068, , ,416, ALA 320 5,068, , ,416, ARG 182 5,811, ,395, ,416, ARG 245 5,811, ,395, ,416, ARG 88 5,811, ,395, ,416, ASP 302 5,562, ,145, ,416, GLN 251 5,614, ,197, ,416, GLN 307 5,614, ,197, ,416, GLU 121 5,665, ,248, ,416, GLU 87 5,665, ,248, ,416, GLY 237 4,965, , ,416, GLY 239 4,965, , ,416, HSD 236 5,659, ,242, ,416, HSD 240 5,659, ,242, ,416, HSD 250 5,659, ,242, ,416, LEU 119 5,378, , ,416, LEU 249 5,378, , ,416, LEU 90 5,378, , ,416, LEU 92 5,378, , ,416, LYS 300 5,524, ,107, ,416, MET 89 6,320, ,903, ,416, PHE 244 5,674, ,258, ,416, Table S min. ALA 252 5,068, , ,416, ALA 320 5,068, , ,416, ARG 182 5,811, ,395, ,416, ARG 245 5,812, ,395, ,416, ARG 308 5,812, ,395, ,416, ARG 88 5,812, ,395, ,416, ASP 302 5,562, ,145, ,416, GLN 307 5,614, ,197, ,416, LEU 90 5,378, , ,416, LEU 92 5,378, , ,416, LYS 300 5,524, ,107, ,416, MET 89 6,322, ,904, ,418, PHE 244 5,675, ,258, ,416, PRO 246 5,272, , ,416, PRO 317 5,272, , ,416, SER 312 5,266, , ,416, SER 91 5,266, , ,416, THR 248 5,369, , ,416, TRP 319 6,020, ,603, ,416, TYR 253 5,872, ,455, ,416, VAL 241 5,275, , ,416, VAL 301 5,275, , ,416, VAL 304 5,275, , ,416,
15 Table S dock. S15 of S19 ALA 128 3,986, , ,333, ALA 320 3,985, , ,333, ALA 322 3,986, , ,333, ARG 144 4,729, ,395, ,333, ARG 182 4,729, ,395, ,333, ARG 211 4,729, ,395, ,333, ASP 125 4,479, ,145, ,333, ASP 295 4,479, ,145, ,333, GLU 121 4,582, ,248, ,333, GLU 184 4,582, ,248, ,333, LEU 122 4,295, , ,333, LEU 129 4,295, , ,333, LEU 90 4,295, , ,333, LEU 92 4,295, , ,333, LYS 123 4,441, ,107, ,333, LYS 300 4,441, ,107, ,333, MET 89 5,237, ,903, ,333, PHE 186 4,592, ,258, ,333, PRO 317 4,188, , ,333, SER 130 4,183, , ,333, SER 264 4,183, , ,333, SER 91 4,183, , ,333, TYR 253 4,789, ,455, ,333, Table S min. GLU 121 4,582, ,248, ,334, ILE 234 4,295, , ,334, ALA 128 3,986, , ,334, ALA 320 3,986, , ,334, ALA 322 3,986, , ,334, ARG 144 4,729, ,395, ,334, ARG 182 4,729, ,395, ,334, ASN 94 4,428, ,094, ,334, ASP 125 4,479, ,145, ,334, CYS 235 5,031, ,697, ,334, LEU 122 4,295, , ,334, LEU 129 4,295, , ,334, LEU 90 4,295, , ,334, LEU 92 4,295, , ,334, LYS 123 4,441, ,107, ,334, LYS 300 4,441, ,107, ,334, MET 89 5,237, ,903, ,334, SER 264 4,183, , ,334, SER 91 4,183, , ,334, TYR 253 4,789, ,455, ,334, VAL 298 4,192, ,583, ,334, GLU 184 4,582, ,248, ,334, GLU 265 4,582, ,248, ,334,
16 Table S dock. S16 of S19 LEU 122 4,295, , ,333, ALA 128 3,985, , ,333, ARG 144 4,729, ,395, ,333, ARG 182 4,729, ,395, ,333, ARG 211 4,729, ,395, ,333, ASP 125 4,479, ,145, ,333, ASP 295 4,479, ,145, ,333, GLU 121 4,582, ,248, ,333, GLU 184 4,582, ,248, ,333, GLU 265 4,582, ,248, ,333, GLY 126 3,882, , ,333, GLY 183 3,882, , ,333, GLY 237 3,882, , ,333, HSD 236 4,576, ,242, ,333, HSD 292 4,576, ,242, ,333, ILE 124 4,294, , ,333, ILE 234 4,295, , ,333, LEU 90 4,295, , ,333, LEU 92 4,295, , ,333, LYS 123 4,441, ,107, ,333, LYS 300 4,441, ,107, ,333, MET 233 5,237, ,903, ,333, MET 89 5,237, ,903, ,333, MET 233 5,237, ,903, ,333, MET 89 5,237, ,903, ,333, Table S min. ILE 234 4,295, , ,334, ALA 128 3,986, , ,334, ALA 322 3,986, , ,334, ARG 144 4,729, ,395, ,334, ARG 182 4,729, ,395, ,334, ARG 211 4,729, ,395, ,334, ASP 125 4,479, ,145, ,334, ASP 295 4,479, ,145, ,334, CYS 235 5,031, ,697, ,334, GLU 121 4,582, ,248, ,334, GLU 184 4,582, ,248, ,334, GLU 265 4,582, ,248, ,334, GLY 126 3,883, , ,334, GLY 183 3,883, , ,334, GLY 237 3,883, , ,334, HSD 236 4,576, ,242, ,334, ILE 124 4,295, , ,334, LEU 122 4,295, , ,334, LEU 90 4,295, , ,334, LEU 92 4,295, , ,334, LYS 123 4,441, ,107, ,334, LYS 300 4,441, ,107, ,334, MET 89 5,237, ,903, ,334,
17 Table S dock. S17 of S19 LEU 238 4,009, , ,048, ALA 252 3,700, , ,048, ALA 320 3,700, , ,048, ARG 182 4,443, ,395, ,048, ARG 308 4,443, ,395, ,048, GLN 307 4,246, ,197, ,048, GLU 121 4,296, ,248, ,048, GLU 265 4,296, ,248, ,048, GLU 87 4,297, ,248, ,048, GLY 237 3,597, , ,048, GLY 239 3,597, , ,048, HSD 236 4,290, ,242, ,048, HSD 240 4,290, ,242, ,048, HSD 250 4,290, ,242, ,048, LEU 119 4,009, , ,048, LEU 249 4,009, , ,048, LEU 256 4,009, , ,048, LEU 90 4,009, , ,048, LEU 92 4,009, , ,048, LYS 300 4,155, ,107, ,048, PRO 246 3,902, , ,048, PRO 317 3,902, , ,048, THR 248 4,000, , ,048, Table S min. LEU 238 4,010, , ,048, ALA 252 3,700, , ,048, ARG 182 4,443, ,395, ,048, ARG 308 4,443, ,395, ,048, CYS 235 4,745, ,697, ,048, GLN 307 4,246, ,197, ,048, GLU 121 4,297, ,248, ,048, GLU 87 4,297, ,248, ,048, GLY 237 3,597, , ,048, GLY 239 3,597, , ,048, HSD 236 4,291, ,242, ,048, HSD 240 4,291, ,242, ,048, HSD 250 4,291, ,242, ,048, LEU 119 4,010, , ,048, LEU 249 4,010, , ,048, LEU 256 4,010, , ,048, LEU 92 4,010, , ,048, LYS 300 4,156, ,107, ,048, PRO 246 3,903, , ,048, PRO 317 3,903, , ,048, THR 248 4,001, , ,048, TRP 319 4,652, ,603, ,048, TYR 253 4,504, ,455, ,048,
18 Table S dock. S18 of S19 ALA 247 5,667, , ,015, ALA 252 5,667, , ,015, ARG 182 6,410, ,395, ,015, ARG 245 6,410, ,395, ,015, ARG 308 6,410, ,395, ,015, ARG 309 6,410, ,395, ,015, ARG 318 6,410, ,395, ,015, ARG 88 6,410, ,395, ,015, LEU 238 5,976, , ,015, LEU 249 5,976, , ,015, LEU 306 5,976, , ,015, LEU 310 5,976, , ,015, LEU 90 5,976, , ,015, LYS 300 6,122, ,107, ,015, MET 89 6,918, ,903, ,015, PHE 244 6,273, ,258, ,015, PRO 246 5,869, , ,015, PRO 317 5,869, , ,015, SER 312 5,864, , ,015, THR 248 5,967, , ,015, TRP 319 6,618, ,603, ,015, TYR 253 6,470, ,455, ,015, VAL 241 5,873, , ,015, Table S min. Amino Acid Etotal (kj/mol) EAA (kj/mol) Eli g (kj/mol) Eint (kj/mol) ALA 252 5,667, , ,015, ARG 182 6,410, ,395, ,015, ARG 245 6,410, ,395, ,015, ARG 308 6,410, ,395, ,015, ARG 309 6,410, ,395, ,015, ARG 88 6,410, ,395, ,015, ASP 302 6,160, ,145, ,015, GLN 307 6,213, ,197, ,015, GLU 242 6,264, ,248, ,015, GLU 87 6,264, ,248, ,015, GLY 237 5,564, , ,015, GLY 239 5,564, , ,015, GLY 311 5,564, , ,015, HSD 236 6,258, ,242, ,015, HSD 240 6,258, ,242, ,015, LEU 238 5,977, , ,015, LEU 249 5,977, , ,015, LEU 310 5,977, , ,015, LEU 90 5,977, , ,015, LYS 300 6,123, ,107, ,015, MET 89 6,919, ,903, ,015, PRO 246 5,870, , ,015, PRO 317 5,870, , ,015,
19 Table S dock. S19 of S19 ALA 247 5,667, , ,015, ALA 252 5,667, , ,015, ARG 182 6,410, ,395, ,015, ARG 245 6,410, ,395, ,015, ARG 308 6,410, ,395, ,015, ARG 309 6,410, ,395, ,015, ARG 318 6,410, ,395, ,015, ARG 88 6,410, ,395, ,015, HSD 250 6,257, ,242, ,015, LEU 238 5,976, , ,015, LEU 249 5,976, , ,015, LEU 306 5,976, , ,015, LEU 310 5,976, , ,015, LEU 90 5,976, , ,015, LYS 300 6,122, ,107, ,015, MET 89 6,918, ,903, ,015, PHE 244 6,273, ,258, ,015, ASP 302 6,160, ,145, ,015, GLN 307 6,213, ,197, ,015, GLU 242 6,263, ,248, ,015, GLU 87 6,263, ,248, ,015, GLY 237 5,564, , ,015, GLY 239 5,564, , ,015,
Comparison of carbon-sulfur and carbon-amine bond in therapeutic drug: -S-aromatic heterocyclic podophyllum derivatives display antitumor activity
 Comparison of carbon-sulfur and carbon-amine bond in therapeutic drug: -S-aromatic heterocyclic podophyllum derivatives display antitumor activity Jian-Long Li 1,a, Wei Zhao 1,a, Chen Zhou 1,a, Ya-Xuan
Comparison of carbon-sulfur and carbon-amine bond in therapeutic drug: -S-aromatic heterocyclic podophyllum derivatives display antitumor activity Jian-Long Li 1,a, Wei Zhao 1,a, Chen Zhou 1,a, Ya-Xuan
Supporting Information
 Supporting Information for Lewis acid-catalyzed redox-neutral amination of 2-(3-pyrroline-1-yl)benzaldehydes via intramolecular [1,5]-hydride shift/isomerization reaction Chun-Huan Jiang, Xiantao Lei,
Supporting Information for Lewis acid-catalyzed redox-neutral amination of 2-(3-pyrroline-1-yl)benzaldehydes via intramolecular [1,5]-hydride shift/isomerization reaction Chun-Huan Jiang, Xiantao Lei,
Supporting Information. Experimental section
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Proton nuclear magnetic resonance ( 1
Highly enantioselective cascade synthesis of spiropyrazolones. Supporting Information. NMR spectra and HPLC traces
 Highly enantioselective cascade synthesis of spiropyrazolones Alex Zea a, Andrea-Nekane R. Alba a, Andrea Mazzanti b, Albert Moyano a and Ramon Rios a,c * Supporting Information NMR spectra and HPLC traces
Highly enantioselective cascade synthesis of spiropyrazolones Alex Zea a, Andrea-Nekane R. Alba a, Andrea Mazzanti b, Albert Moyano a and Ramon Rios a,c * Supporting Information NMR spectra and HPLC traces
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Trichodermides A E: New Peptaibols isolated from Australian Termite Nestderived Fungus Trichoderma virens CMB-TN16 Wei-Hua Jiao,, Zeinab Khalil, Pradeep Dewapriya, Angela A. Salim,
SUPPORTING INFORMATION Trichodermides A E: New Peptaibols isolated from Australian Termite Nestderived Fungus Trichoderma virens CMB-TN16 Wei-Hua Jiao,, Zeinab Khalil, Pradeep Dewapriya, Angela A. Salim,
Supporting Information. Asymmetric Binary-acid Catalysis with Chiral. Phosphoric Acid and MgF 2 : Catalytic
 Supporting Information Asymmetric Binary-acid Catalysis with Chiral Phosphoric Acid and MgF 2 : Catalytic Enantioselective Friedel-Crafts Reactions of β,γ- Unsaturated-α-Ketoesters Jian Lv, Xin Li, Long
Supporting Information Asymmetric Binary-acid Catalysis with Chiral Phosphoric Acid and MgF 2 : Catalytic Enantioselective Friedel-Crafts Reactions of β,γ- Unsaturated-α-Ketoesters Jian Lv, Xin Li, Long
A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 A facile and general route to 3-((trifluoromethyl)thio)benzofurans and 3-((trifluoromethyl)thio)benzothiophenes
Supporting Information One-Pot Approach to Chiral Chromenes via Enantioselective Organocatalytic Domino Oxa-Michael-Aldol Reaction
 Supporting Information ne-pot Approach to Chiral Chromenes via Enantioselective rganocatalytic Domino xa-michael-aldol Reaction Hao Li, Jian Wang, Timiyin E-Nunu, Liansuo Zu, Wei Jiang, Shaohua Wei, *
Supporting Information ne-pot Approach to Chiral Chromenes via Enantioselective rganocatalytic Domino xa-michael-aldol Reaction Hao Li, Jian Wang, Timiyin E-Nunu, Liansuo Zu, Wei Jiang, Shaohua Wei, *
The effect of curcumin on the stability of Aβ. dimers
 The effect of curcumin on the stability of Aβ dimers Li Na Zhao, See-Wing Chiu, Jérôme Benoit, Lock Yue Chew,, and Yuguang Mu, School of Physical and Mathematical Sciences, Nanyang Technological University,
The effect of curcumin on the stability of Aβ dimers Li Na Zhao, See-Wing Chiu, Jérôme Benoit, Lock Yue Chew,, and Yuguang Mu, School of Physical and Mathematical Sciences, Nanyang Technological University,
ÂÓÈÎ ÁÈ ÙÔ K ÙÙ ÚÔ 1 Ô KÂÊ Ï ÈÔ 1.1 E Ë Î ÙÙ ÚˆÓ 1.1.1 ÚÔÎ Ú ˆÙÈÎ Î ÙÙ Ú
 11 1 Ô KÂÊ Ï ÈÔ ÂÓÈÎ ÁÈ ÙÔ K ÙÙ ÚÔ 1.1 E Ë Î ÙÙ ÚˆÓ Στο κεφάλαιο αυτό θα αναφερθούμε σύντομα στο κύτταρο, τα είδη (ευκαρυωτικά και προκαρυωτικά) και γενικά στα διάφορα στοιχεία του, όπως πυρήνα, κυτταρόπλασμα
11 1 Ô KÂÊ Ï ÈÔ ÂÓÈÎ ÁÈ ÙÔ K ÙÙ ÚÔ 1.1 E Ë Î ÙÙ ÚˆÓ Στο κεφάλαιο αυτό θα αναφερθούμε σύντομα στο κύτταρο, τα είδη (ευκαρυωτικά και προκαρυωτικά) και γενικά στα διάφορα στοιχεία του, όπως πυρήνα, κυτταρόπλασμα
ΑΝΤΙΓΡΑΦΗ ΚΑΙ ΕΚΦΡΑΣΗ ΤΗΣ ΓΕΝΕΤΙΚΗΣ ΠΛΗΡΟΦΟΡΙΑΣ
 ΜΙΝΟΠΕΤΡΟΣ ΚΩΝΣΤΑΝΤΙΝΟΣ ΦΥΣΙΚΟΣ - Ρ/Η ΚΑΘΗΓΗΤΗΣ ΕΝΙΑΙΟΥ ΛΥΚΕΙΟΥ ΥΠΕΥΘΥΝΟΣ ΣΕΦΕ 2 ου ΕΝΙΑΙΟΥ ΛΥΚΕΙΟΥ ΠΕΡΑΜΑΤΟΣ ΕΡΓΑΣΤΗΡΙΑΚΗ ΑΣΚΗΣΗ ΒΙΟΛΟΓΙΑΣ ΚΑΤΕΥΘΥΝΣΗΣ Γ ΛΥΚΕΙΟΥ ΑΝΤΙΓΡΑΦΗ ΚΑΙ ΕΚΦΡΑΣΗ ΤΗΣ ΓΕΝΕΤΙΚΗΣ ΠΛΗΡΟΦΟΡΙΑΣ
ΜΙΝΟΠΕΤΡΟΣ ΚΩΝΣΤΑΝΤΙΝΟΣ ΦΥΣΙΚΟΣ - Ρ/Η ΚΑΘΗΓΗΤΗΣ ΕΝΙΑΙΟΥ ΛΥΚΕΙΟΥ ΥΠΕΥΘΥΝΟΣ ΣΕΦΕ 2 ου ΕΝΙΑΙΟΥ ΛΥΚΕΙΟΥ ΠΕΡΑΜΑΤΟΣ ΕΡΓΑΣΤΗΡΙΑΚΗ ΑΣΚΗΣΗ ΒΙΟΛΟΓΙΑΣ ΚΑΤΕΥΘΥΝΣΗΣ Γ ΛΥΚΕΙΟΥ ΑΝΤΙΓΡΑΦΗ ΚΑΙ ΕΚΦΡΑΣΗ ΤΗΣ ΓΕΝΕΤΙΚΗΣ ΠΛΗΡΟΦΟΡΙΑΣ
a 2,5 b 2,5 upplemental Figure 1 IL4 (4h) in Ja18-/- mice IFN-γ (16h) in Ja18-/- mice 1,5 1,5 ng/ml ng/ml 0,5 0,5 4ClPh PyrC 4ClPh PyrC
 a 2,5 IL4 (4h) in Ja18-/- mice b 2,5 IFN-γ (16h) in Ja18-/- mice 2 2 ng/ml 1,5 ng/ml 1,5 1 1,5,5 4ClPh NC PyrC 4ClPh NC PyrC upplemental Figure 1 a b c PyrC-α-GalCer NU-α-GalCer α-galcer CD4 (PE) MFI CD4
a 2,5 IL4 (4h) in Ja18-/- mice b 2,5 IFN-γ (16h) in Ja18-/- mice 2 2 ng/ml 1,5 ng/ml 1,5 1 1,5,5 4ClPh NC PyrC 4ClPh NC PyrC upplemental Figure 1 a b c PyrC-α-GalCer NU-α-GalCer α-galcer CD4 (PE) MFI CD4
Targeting Bacillus anthracis toxicity with a genetically selected inhibitor of the PA/CMG2
 Supplemental Information Targeting Bacillus anthracis toxicity with a genetically selected inhibitor of the PA/CMG2 protein-protein interaction. Abigail L. Male, 1 Fedor Forafonov, 1 Francesco Cuda, 1
Supplemental Information Targeting Bacillus anthracis toxicity with a genetically selected inhibitor of the PA/CMG2 protein-protein interaction. Abigail L. Male, 1 Fedor Forafonov, 1 Francesco Cuda, 1
Electronic Supplementary Information
 Electronic Supplementary Information NbCl 3 -catalyzed [2+2+2] intermolecular cycloaddition of alkynes and alkenes to 1,3-cyclohexadiene derivatives Yasushi Obora,* Keisuke Takeshita and Yasutaka Ishii*
Electronic Supplementary Information NbCl 3 -catalyzed [2+2+2] intermolecular cycloaddition of alkynes and alkenes to 1,3-cyclohexadiene derivatives Yasushi Obora,* Keisuke Takeshita and Yasutaka Ishii*
Supporting Information
 Supporting Information Wiley-VC 007 9 Weinheim, Germany ew ear Infrared Dyes and Fluorophores Based on Diketopyrrolopyrroles Dipl.-Chem. Georg M. Fischer, Dipl.-Chem. Andreas P. Ehlers, Prof. Dr. Andreas
Supporting Information Wiley-VC 007 9 Weinheim, Germany ew ear Infrared Dyes and Fluorophores Based on Diketopyrrolopyrroles Dipl.-Chem. Georg M. Fischer, Dipl.-Chem. Andreas P. Ehlers, Prof. Dr. Andreas
Supporting Information. Copyright Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2007
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2007 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for High-Throughput Substrate
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2007 Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2006 Supporting Information for High-Throughput Substrate
Metal-free Oxidative Coupling of Amines with Sodium Sulfinates: A Mild Access to Sulfonamides
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting information for Metal-free Oxidative Coupling of Amines with Sodium Sulfinates:
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting information for Metal-free Oxidative Coupling of Amines with Sodium Sulfinates:
Vilsmeier Haack reagent-promoted formyloxylation of α-chloro-narylacetamides
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 205 Vilsmeier aack reagent-promoted formyloxylation of α-chloro-arylacetamides by formamide Jiann-Jyh
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 205 Vilsmeier aack reagent-promoted formyloxylation of α-chloro-arylacetamides by formamide Jiann-Jyh
SUPPLEMENTARY MATERIAL
 10.1071/CH16014_AC The Authors 2016 Australian Journal of Chemistry 2016, 69(9), 1049-1053 SUPPLEMENTARY MATERIAL A Green Approach for the Synthesis of Novel 7,11-Dihydro-6H-chromeno[3,4- e]isoxazolo[5,4-b]pyridin-6-one
10.1071/CH16014_AC The Authors 2016 Australian Journal of Chemistry 2016, 69(9), 1049-1053 SUPPLEMENTARY MATERIAL A Green Approach for the Synthesis of Novel 7,11-Dihydro-6H-chromeno[3,4- e]isoxazolo[5,4-b]pyridin-6-one
Effects of feeding reduced dietary CP with supplementation of synthetic AA on N and C. excretion, energy utilization, and fecal VFA concentrations.
 Effects of feeding reduced dietary CP with supplementation of synthetic AA on N and C excretion, energy utilization, and fecal VFA concentrations. Aaron Jones, Brian Richert, Charlie Maxwell, Scott Radcliffe
Effects of feeding reduced dietary CP with supplementation of synthetic AA on N and C excretion, energy utilization, and fecal VFA concentrations. Aaron Jones, Brian Richert, Charlie Maxwell, Scott Radcliffe
Copper-Catalyzed Oxidative Dehydrogenative N-N Bond. Formation for the Synthesis of N,N -Diarylindazol-3-ones
 Electronic Supplementary Material (ESI) for Organic Chemistry Frontiers. This journal is the Partner Organisations 2016 Supporting information Copper-Catalyzed Oxidative Dehydrogenative - Bond Formation
Electronic Supplementary Material (ESI) for Organic Chemistry Frontiers. This journal is the Partner Organisations 2016 Supporting information Copper-Catalyzed Oxidative Dehydrogenative - Bond Formation
4 ο ΚΕΦΑΛΑΙΟ. Γ ε ν ε τ ι κ ή
 4 ο ΚΕΦΑΛΑΙΟ Γ ε ν ε τ ι κ ή 1. Κύκλος της ζωής του κυττάρου 3ο Γελ. Ηλιούπολης επιμέλεια: Αργύρης Γιάννης 2 2. Μοριακή Γενετική i). Ροή της γενετικής πληροφορίας DNA RNA πρωτεΐνες νουκλεΐκά οξέα ή πρωτεΐνες
4 ο ΚΕΦΑΛΑΙΟ Γ ε ν ε τ ι κ ή 1. Κύκλος της ζωής του κυττάρου 3ο Γελ. Ηλιούπολης επιμέλεια: Αργύρης Γιάννης 2 2. Μοριακή Γενετική i). Ροή της γενετικής πληροφορίας DNA RNA πρωτεΐνες νουκλεΐκά οξέα ή πρωτεΐνες
Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3
 Supporting Information Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3 Shohei Shimokawa, Yuhsuke Kawagoe, Katsuhiko Moriyama, Hideo Togo* Graduate
Supporting Information Direct Transformation of Ethylarenes into Primary Aromatic Amides with N-Bromosuccinimide and I 2 -aq NH 3 Shohei Shimokawa, Yuhsuke Kawagoe, Katsuhiko Moriyama, Hideo Togo* Graduate
SUPPORTING INFORMATION
 SUPPRTING INFRMATIN Per(-guanidino--deoxy)cyclodextrins: Synthesis, characterisation and binding behaviour toward selected small molecules and DNA. Nikolaos Mourtzis, a Kyriaki Eliadou, a Chrysie Aggelidou,
SUPPRTING INFRMATIN Per(-guanidino--deoxy)cyclodextrins: Synthesis, characterisation and binding behaviour toward selected small molecules and DNA. Nikolaos Mourtzis, a Kyriaki Eliadou, a Chrysie Aggelidou,
Electronic Supplementary Information
 Electronic Supplementary Information The preferred all-gauche conformations in 3-fluoro-1,2-propanediol Laize A. F. Andrade, a Josué M. Silla, a Claudimar J. Duarte, b Roberto Rittner, b Matheus P. Freitas*,a
Electronic Supplementary Information The preferred all-gauche conformations in 3-fluoro-1,2-propanediol Laize A. F. Andrade, a Josué M. Silla, a Claudimar J. Duarte, b Roberto Rittner, b Matheus P. Freitas*,a
Ζεύγη βάσεων ΓΕΝΕΤΙΚΗ. Γουανίνη Κυτοσίνη. 4α. Λειτουργία γενετικού υλικού. Φωσφοδιεστερικός δεσμός
 εύγη βάσεων Αδενίνη Θυμίνη Γουανίνη Κυτοσίνη ΓΕΝΕΤΙΚΗ Φωσφοδιεστερικός δεσμός 4α. Λειτουργία γενετικού υλικού 1 ΛΕΙΤΟΥΡΓΙΑ ΓΕΝΕΤΙΚΟΥ ΥΛΙΚΟΥ Αντιγραφή (διπλασιασμός) DNA: DNA DNA Έκφραση γενετικής πληροφορίας:
εύγη βάσεων Αδενίνη Θυμίνη Γουανίνη Κυτοσίνη ΓΕΝΕΤΙΚΗ Φωσφοδιεστερικός δεσμός 4α. Λειτουργία γενετικού υλικού 1 ΛΕΙΤΟΥΡΓΙΑ ΓΕΝΕΤΙΚΟΥ ΥΛΙΚΟΥ Αντιγραφή (διπλασιασμός) DNA: DNA DNA Έκφραση γενετικής πληροφορίας:
Direct Palladium-Catalyzed Arylations of Aryl Bromides. with 2/9-Substituted Pyrimido[5,4-b]indolizines
![Direct Palladium-Catalyzed Arylations of Aryl Bromides. with 2/9-Substituted Pyrimido[5,4-b]indolizines Direct Palladium-Catalyzed Arylations of Aryl Bromides. with 2/9-Substituted Pyrimido[5,4-b]indolizines](/thumbs/87/95187440.jpg) Direct Palladium-Catalyzed Arylations of Aryl Bromides with 2/9-Substituted Pyrimido[5,4-b]indolizines Min Jiang, Ting Li, Linghua Meng, Chunhao Yang,* Yuyuan Xie*, and Jian Ding State Key Laboratory of
Direct Palladium-Catalyzed Arylations of Aryl Bromides with 2/9-Substituted Pyrimido[5,4-b]indolizines Min Jiang, Ting Li, Linghua Meng, Chunhao Yang,* Yuyuan Xie*, and Jian Ding State Key Laboratory of
Αµινοξέα και πεπτίδια Φύλλο εργασίας - αξιολόγησης
 Αµινοξέα και πεπτίδια Φύλλο εργασίας - αξιολόγησης Τάξη. Ονοµατεπώνυµο Μάθηµα Γνωστικό αντικείµενο: Χηµεία,Βιοχηµεία......................... ιδακτική ενότητα Αµινοξέα και πεπτίδια Τµήµα........... Απαιτούµενος
Αµινοξέα και πεπτίδια Φύλλο εργασίας - αξιολόγησης Τάξη. Ονοµατεπώνυµο Μάθηµα Γνωστικό αντικείµενο: Χηµεία,Βιοχηµεία......................... ιδακτική ενότητα Αµινοξέα και πεπτίδια Τµήµα........... Απαιτούµενος
Rhodium-Catalyzed Oxidative Decarbonylative Heck-type Coupling of Aromatic Aldehydes with Terminal Alkenes
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Rhodium-Catalyzed Oxidative Decarbonylative Heck-type
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Rhodium-Catalyzed Oxidative Decarbonylative Heck-type
Supporting Information. Experimental section
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Anhydrous solvents were transferred by
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supporting Information Experimental section General. Anhydrous solvents were transferred by
Supplementary Materials
 Supplementary Materials Figure S1. Chemical structures of phloracetophenone intermediates (2a, 2c 2g). 2a 2c 2d 2e 2f 2g Figure S2. Chemical structures of geranylacetophenone analogues (3a, 3c, 3e g).
Supplementary Materials Figure S1. Chemical structures of phloracetophenone intermediates (2a, 2c 2g). 2a 2c 2d 2e 2f 2g Figure S2. Chemical structures of geranylacetophenone analogues (3a, 3c, 3e g).
Supplementary!Information!
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015! Synthesis)and)characterisation)of)an)open1cage) fullerene)encapsulating)hydrogen)fluoride! Supplementary!Information!
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015! Synthesis)and)characterisation)of)an)open1cage) fullerene)encapsulating)hydrogen)fluoride! Supplementary!Information!
ΗΜΟΚΡΙΤΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΡΑΚΗΣ ΤΜΗΜΑ ΜΟΡΙΑΚΗΣ ΒΙΟΛΟΓΙΑΣ ΚΑΙ ΓΕΝΕΤΙΚΗΣ ΓΟΝΙ ΙΑΚΗ ΕΚΦΡΑΣΗ ΚΑΙ ΣΗΜΑΤΟ ΟΤΗΣΗ
 ΗΜΟΚΡΙΤΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΡΑΚΗΣ ΤΜΗΜΑ ΜΟΡΙΑΚΗΣ ΒΙΟΛΟΓΙΑΣ ΚΑΙ ΓΕΝΕΤΙΚΗΣ ΓΟΝΙ ΙΑΚΗ ΕΚΦΡΑΣΗ ΚΑΙ ΣΗΜΑΤΟ ΟΤΗΣΗ ρ. Α. ΓΑΛΑΝΗΣ agalanis@mbg.duth.gr Figure 6.1 The Biology of Cancer ( Garland Science 2007) Figure
ΗΜΟΚΡΙΤΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΡΑΚΗΣ ΤΜΗΜΑ ΜΟΡΙΑΚΗΣ ΒΙΟΛΟΓΙΑΣ ΚΑΙ ΓΕΝΕΤΙΚΗΣ ΓΟΝΙ ΙΑΚΗ ΕΚΦΡΑΣΗ ΚΑΙ ΣΗΜΑΤΟ ΟΤΗΣΗ ρ. Α. ΓΑΛΑΝΗΣ agalanis@mbg.duth.gr Figure 6.1 The Biology of Cancer ( Garland Science 2007) Figure
Electronic Supplementary Information. Carbon dioxide as a reversible amine-protecting
 Electronic Supplementary Information Carbon dioxide as a reversible amine-protecting agent in selective Michael additions and acylations Annelies Peeters, Rob Ameloot and Dirk E. De Vos * Centre for Surface
Electronic Supplementary Information Carbon dioxide as a reversible amine-protecting agent in selective Michael additions and acylations Annelies Peeters, Rob Ameloot and Dirk E. De Vos * Centre for Surface
Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic acids with the assistance of isocyanide
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Copper-catalyzed formal O-H insertion reaction of α-diazo-1,3-dicarb- onyl compounds to carboxylic
Patrycja Miszczyk, Dorota Wieczorek, Joanna Gałęzowska, Błażej Dziuk, Joanna Wietrzyk and Ewa Chmielewska. 1. Spectroscopic Data.
 ; doi:10.3390/molecules22020254 S1 of S23 Supplementary Materials: Reaction of 3-Amino-1,2,4-Triazole with Diethyl Phosphite and Triethyl Orthoformate: Acid-Base Properties and Antiosteoporotic Activities
; doi:10.3390/molecules22020254 S1 of S23 Supplementary Materials: Reaction of 3-Amino-1,2,4-Triazole with Diethyl Phosphite and Triethyl Orthoformate: Acid-Base Properties and Antiosteoporotic Activities
Comparison of nutrient components in muscle of wild and farmed groups of Myxocyprinus asiaticus
 41 6 Vol. 41 No. 6 Freshwater Fisheries 2011 11 Nov. 2011 430070 447. 5 ± 20. 5 g 484. 3 ± 19. 6 g Myxocyprinus asiaticus P < 0. 05 P < 0. 05 17 EAAI 70. 39 67. 02 AAS Val CS Met + Cys ΣSFA 44. 23% 31.
41 6 Vol. 41 No. 6 Freshwater Fisheries 2011 11 Nov. 2011 430070 447. 5 ± 20. 5 g 484. 3 ± 19. 6 g Myxocyprinus asiaticus P < 0. 05 P < 0. 05 17 EAAI 70. 39 67. 02 AAS Val CS Met + Cys ΣSFA 44. 23% 31.
Οι πρωτεΐνες δομούνται από ένα σύνολο αμινοξέων. 1/10/2015 Δ.Δ. Λεωνίδας
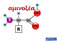 αμινοξέα Οι πρωτεΐνες δομούνται από ένα σύνολο αμινοξέων Λυσίνη CORN Ισομερές L Ισομερές D R = πλευρική αλυσίδα (side chain) Τα περισσότερα αμινοξέα είναι ασύμμετρα Όλα τα αμινοξέα που βρίσκονται στις
αμινοξέα Οι πρωτεΐνες δομούνται από ένα σύνολο αμινοξέων Λυσίνη CORN Ισομερές L Ισομερές D R = πλευρική αλυσίδα (side chain) Τα περισσότερα αμινοξέα είναι ασύμμετρα Όλα τα αμινοξέα που βρίσκονται στις
Supplementary Materials for. Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides
 Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Table of Contents 1 Supplementary Data MCD
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2017 Supporting Information for Magnetic circular dichroism and density functional theory
1.1 1., Litopenaeus vannamei N- -β-d- NAGase. Asp Glu. K I 9.50 mmol/l mmol/l. Litopenaeus vannamei
 N--β-D- 1, 2 1 1 1., 361005 2. 362000 GlyAlaValLeu Ile Pro PheTrpSerThr Cys Gln AsnGlu AspHis LysArg 18 Litopenaeus vannamei N--β-D- pnp-nag NAGase L-His Lys Arg Asp Glu IC 50 20 28 mmol/l Asp Glu pnp-nag
N--β-D- 1, 2 1 1 1., 361005 2. 362000 GlyAlaValLeu Ile Pro PheTrpSerThr Cys Gln AsnGlu AspHis LysArg 18 Litopenaeus vannamei N--β-D- pnp-nag NAGase L-His Lys Arg Asp Glu IC 50 20 28 mmol/l Asp Glu pnp-nag
Electronic Supporting Information. 3-Aminothiophenecarboxylic acid (3-Atc)-induced folding in peptides
 Electronic Supplementary Material (ESI for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 Electronic Supporting Information
Electronic Supplementary Material (ESI for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 Electronic Supporting Information
Enantioselective Organocatalytic Michael Addition of Isorhodanines. to α, β-unsaturated Aldehydes
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Enantioselective Organocatalytic Michael Addition of Isorhodanines to α,
Supporting Information
 Supporting Information Lewis acid catalyzed ring-opening reactions of methylenecyclopropanes with diphenylphosphine oxide in the presence of sulfur or selenium Min Shi,* Min Jiang and Le-Ping Liu State
Supporting Information Lewis acid catalyzed ring-opening reactions of methylenecyclopropanes with diphenylphosphine oxide in the presence of sulfur or selenium Min Shi,* Min Jiang and Le-Ping Liu State
First Total Synthesis of Antimitotic Compound, (+)-Phomopsidin
 First Total Synthesis of Antimitotic Compound, (+)-Phomopsidin Takahiro Suzuki, a Kenji Usui, a Yoshiharu Miyake, a Michio Namikoshi, b and Masahisa Nakada a, * a Department of Chemistry, School of Science
First Total Synthesis of Antimitotic Compound, (+)-Phomopsidin Takahiro Suzuki, a Kenji Usui, a Yoshiharu Miyake, a Michio Namikoshi, b and Masahisa Nakada a, * a Department of Chemistry, School of Science
Human angiogenin is a potent cytotoxin in the absence of ribonuclease inhibitor
 Human angiogenin is a potent cytotoxin in the absence of ribonuclease inhibitor SYDNEY P. THOMAS, 1 TRISH T. HOANG, 2 VALERIE T. RESSLER, 4 and RONALD T. RAINES 2,3,4 1 Graduate Program in Cell & Molecular
Human angiogenin is a potent cytotoxin in the absence of ribonuclease inhibitor SYDNEY P. THOMAS, 1 TRISH T. HOANG, 2 VALERIE T. RESSLER, 4 and RONALD T. RAINES 2,3,4 1 Graduate Program in Cell & Molecular
Synthesis of Imines from Amines in Aliphatic Alcohols on Pd/ZrO 2 Catalyst at Ambient Conditions
 This journal is The Royal Society of Chemistry 213 Synthesis of Imines from Amines in Aliphatic Alcohols on Pd/ZrO 2 Catalyst at Ambient Conditions Wenjing Cui, a Bao Zhaorigetu,* a Meilin Jia, a and Wulan
This journal is The Royal Society of Chemistry 213 Synthesis of Imines from Amines in Aliphatic Alcohols on Pd/ZrO 2 Catalyst at Ambient Conditions Wenjing Cui, a Bao Zhaorigetu,* a Meilin Jia, a and Wulan
Supporting Information
 Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2014 A Family of Click Nucleosides for Metal-Mediated Base Pairing: Unravelling the Principles of Highly Stabilizing Metal-Mediated
Supporting Information Copyright Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, 2014 A Family of Click Nucleosides for Metal-Mediated Base Pairing: Unravelling the Principles of Highly Stabilizing Metal-Mediated
Phosphorus Oxychloride as an Efficient Coupling Reagent for the Synthesis of Ester, Amide and Peptide under Mild Conditions
 Supplementary Information for Phosphorus xychloride as an Efficient Coupling Reagent for the Synthesis of Ester, Amide and Peptide under Mild Conditions u Chen,* a,b Xunfu Xu, a Liu Liu, a Guo Tang,* a
Supplementary Information for Phosphorus xychloride as an Efficient Coupling Reagent for the Synthesis of Ester, Amide and Peptide under Mild Conditions u Chen,* a,b Xunfu Xu, a Liu Liu, a Guo Tang,* a
Supporting Information
 Electronic upplementary Material (EI) for Green Chemistry. This journal is The Royal ociety of Chemistry 204 upporting Information ynthesis of sulfonamides via I 2 -mediated reaction of sodium sulfinates
Electronic upplementary Material (EI) for Green Chemistry. This journal is The Royal ociety of Chemistry 204 upporting Information ynthesis of sulfonamides via I 2 -mediated reaction of sodium sulfinates
Supporting Information
 Supporting Information for AgOTf-catalyzed one-pot reactions of 2-alkynylbenzaldoximes with α,β-unsaturated carbonyl compounds Qiuping Ding 1, Dan Wang 1, Puying Luo* 2, Meiling Liu 1, Shouzhi Pu* 3 and
Supporting Information for AgOTf-catalyzed one-pot reactions of 2-alkynylbenzaldoximes with α,β-unsaturated carbonyl compounds Qiuping Ding 1, Dan Wang 1, Puying Luo* 2, Meiling Liu 1, Shouzhi Pu* 3 and
9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer catalysts
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2014 9-amino-(9-deoxy)cinchona alkaloids-derived novel chiral phase-transfer
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Modular Synthesis of Propargylamine Modified Cyclodextrins by a Gold(III)-catalyzed Three Component
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Modular Synthesis of Propargylamine Modified Cyclodextrins by a Gold(III)-catalyzed Three Component
Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes
![Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes](/thumbs/92/108867714.jpg) SUPPORTING INFORMATION Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Eric J. Uzelac, Casey B. McCausland, and Seth C. Rasmussen*
SUPPORTING INFORMATION Pyrrolo[2,3-d:5,4-d']bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes Eric J. Uzelac, Casey B. McCausland, and Seth C. Rasmussen*
Divergent synthesis of various iminocyclitols from D-ribose
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 205 Divergent synthesis of various iminocyclitols from D-ribose Ramu Petakamsetty,
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 205 Divergent synthesis of various iminocyclitols from D-ribose Ramu Petakamsetty,
Supporting Information. Consecutive hydrazino-ugi-azide reactions: synthesis of acylhydrazines bearing 1,5- disubstituted tetrazoles
 Supporting Information for Consecutive hydrazino-ugi-azide reactions: synthesis of acylhydrazines bearing 1,5- disubstituted tetrazoles Angélica de Fátima S. Barreto*, Veronica Alves dos Santos, and Carlos
Supporting Information for Consecutive hydrazino-ugi-azide reactions: synthesis of acylhydrazines bearing 1,5- disubstituted tetrazoles Angélica de Fátima S. Barreto*, Veronica Alves dos Santos, and Carlos
Τάξη. Γνωστικό αντικείµενο: Ειδικοί διδακτικοί στόχοι
 Αµινοξέα και πεπτίδια Τάξη Μάθηµα Γνωστικό αντικείµενο: ιδακτική ενότητα Απαιτούµενος χρόνος Χηµεία,Βιοχηµεία. Αµινοξέα και πεπτίδια 2 διδακτικές ώρες Ειδικοί διδακτικοί στόχοι Οι διδακτικοί στόχοι αυτών
Αµινοξέα και πεπτίδια Τάξη Μάθηµα Γνωστικό αντικείµενο: ιδακτική ενότητα Απαιτούµενος χρόνος Χηµεία,Βιοχηµεία. Αµινοξέα και πεπτίδια 2 διδακτικές ώρες Ειδικοί διδακτικοί στόχοι Οι διδακτικοί στόχοι αυτών
Supporting Information. A catalyst-free multicomponent domino sequence for the. diastereoselective synthesis of (E)-3-[2-arylcarbonyl-3-
 Supporting Information for A catalyst-free multicomponent domino sequence for the diastereoselective synthesis of (E)-3-[2-arylcarbonyl-3- (arylamino)allyl]chromen-4-ones Pitchaimani Prasanna 1, Pethaiah
Supporting Information for A catalyst-free multicomponent domino sequence for the diastereoselective synthesis of (E)-3-[2-arylcarbonyl-3- (arylamino)allyl]chromen-4-ones Pitchaimani Prasanna 1, Pethaiah
Supporting Information
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Crotonols A and B, Two Rare Tigliane Diterpenoid
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2018 Supporting Information Crotonols A and B, Two Rare Tigliane Diterpenoid
Improved Peptide and Protein Torsional Energetics with the OPLS-AA Force Field
 Improved Peptide and Protein Torsional Energetics with the OPLS-AA Force Field Supplementary Information Michael J. Robertson, Julian Tirado-Rives, and William L. Jorgensen* Department of Chemistry, Yale
Improved Peptide and Protein Torsional Energetics with the OPLS-AA Force Field Supplementary Information Michael J. Robertson, Julian Tirado-Rives, and William L. Jorgensen* Department of Chemistry, Yale
Kishore Natte, Jianbin Chen, Helfried Neumann, Matthias Beller, and Xiao-Feng Wu*
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 204 Kishore Natte, Jianbin Chen, Helfried Neumann, Matthias Beller, and Xiao-Feng
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 204 Kishore Natte, Jianbin Chen, Helfried Neumann, Matthias Beller, and Xiao-Feng
Supporting Information
 Supporting Information Synthesis and Electroactive Properties of Poly(amidoamine) Dendrimers with an Aniline Pentamer Shell Wei-I Hung a, Chih-Bing Hung a, Ya-Han Chang a, Jiun-Kuang Dai a, Yan Li b, Hai
Supporting Information Synthesis and Electroactive Properties of Poly(amidoamine) Dendrimers with an Aniline Pentamer Shell Wei-I Hung a, Chih-Bing Hung a, Ya-Han Chang a, Jiun-Kuang Dai a, Yan Li b, Hai
Design and Solid Phase Synthesis of New DOTA Conjugated (+)-Biotin Dimers Planned to Develop Molecular Weight-Tuned Avidin Oligomers
 Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2015 Supplementary Information Design and Solid Phase Synthesis of New DTA Conjugated
Electronic Supplementary Material (ESI) for rganic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2015 Supplementary Information Design and Solid Phase Synthesis of New DTA Conjugated
difluoroboranyls derived from amides carrying donor group Supporting Information
 The influence of the π-conjugated spacer on photophysical properties of difluoroboranyls derived from amides carrying donor group Supporting Information Anna Maria Grabarz a Adèle D. Laurent b, Beata Jędrzejewska
The influence of the π-conjugated spacer on photophysical properties of difluoroboranyls derived from amides carrying donor group Supporting Information Anna Maria Grabarz a Adèle D. Laurent b, Beata Jędrzejewska
BIOXHMEIA, TOMOΣ I ΠANEΠIΣTHMIAKEΣ EKΔOΣEIΣ KPHTHΣ ΑΠΕΙΚΟΝΙΣΗ ΜΟΡΙΑΚΩΝ ΔΟΜΩΝ
 BIOXHMEIA, TOMOΣ I ΠANEΠIΣTHMIAKEΣ EKΔOΣEIΣ KPHTHΣ ΑΠΕΙΚΟΝΙΣΗ ΜΟΡΙΑΚΩΝ ΔΟΜΩΝ ΑΠΕΙΚΟΝΙΣΗ ΜΟΡΙΑΚΩΝ ΔΟΜΩΝ ΜΟΡΙΑΚΑ ΜΟΝΤΕΛΑ 1: ΧΩΡΟΠΛΗΡΩΤΙΚΟ ΜΟΝΤΕΛΟ (SPACE-FILLING) 1: ΧΩΡΟΠΛΗΡΩΤΙΚΟ ΜΟΝΤΕΛΟ (SPACE-FILLING)
BIOXHMEIA, TOMOΣ I ΠANEΠIΣTHMIAKEΣ EKΔOΣEIΣ KPHTHΣ ΑΠΕΙΚΟΝΙΣΗ ΜΟΡΙΑΚΩΝ ΔΟΜΩΝ ΑΠΕΙΚΟΝΙΣΗ ΜΟΡΙΑΚΩΝ ΔΟΜΩΝ ΜΟΡΙΑΚΑ ΜΟΝΤΕΛΑ 1: ΧΩΡΟΠΛΗΡΩΤΙΚΟ ΜΟΝΤΕΛΟ (SPACE-FILLING) 1: ΧΩΡΟΠΛΗΡΩΤΙΚΟ ΜΟΝΤΕΛΟ (SPACE-FILLING)
 1 ΕΠΙΣΤΗΜΗ ΚΑΙ ΜΗΧΑΝΙΚΗ ΒΙΟΛΟΓΙΚΩΝ ΣΥΣΤΗΜΑΤΩΝ ΚΑΙ ΠΡΟΪΟΝΤΩΝ (ΤΡΟΦΙΜΑ-ΒΙΟΤΕΧΝΟΛΟΓΙΑ) Περιεχόµενο - Σκοπός του Μαθήµατος 6 ο ΕΞΑΜΗΝΟ ΧΗΜΙΚΩΝ ΜΗΧΑΝΙΚΩΝ Ο ΗΓΙΕΣ ΜΑΘΗΜΑΤΟΣ Το µάθηµα Επιστήµη και Μηχανική Βιολογικών
1 ΕΠΙΣΤΗΜΗ ΚΑΙ ΜΗΧΑΝΙΚΗ ΒΙΟΛΟΓΙΚΩΝ ΣΥΣΤΗΜΑΤΩΝ ΚΑΙ ΠΡΟΪΟΝΤΩΝ (ΤΡΟΦΙΜΑ-ΒΙΟΤΕΧΝΟΛΟΓΙΑ) Περιεχόµενο - Σκοπός του Μαθήµατος 6 ο ΕΞΑΜΗΝΟ ΧΗΜΙΚΩΝ ΜΗΧΑΝΙΚΩΝ Ο ΗΓΙΕΣ ΜΑΘΗΜΑΤΟΣ Το µάθηµα Επιστήµη και Μηχανική Βιολογικών
Supporting Information
 Supporting Information Metal-catalyzed Stereoselective and Protecting-group-free Synthesis of 1,2-cis-Glycosides Using 4,6-Dimethoxy-1,3,5-triazin-2-yl Glycosides as Glycosyl Donors Tomonari Tanaka,* 1
Supporting Information Metal-catalyzed Stereoselective and Protecting-group-free Synthesis of 1,2-cis-Glycosides Using 4,6-Dimethoxy-1,3,5-triazin-2-yl Glycosides as Glycosyl Donors Tomonari Tanaka,* 1
Synthesis, structural studies and stability of the model, cysteine containing DNA-protein cross-links
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 ELECTRONIC SUPPLEMENTARY INFORMATION
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 ELECTRONIC SUPPLEMENTARY INFORMATION
Supporting Information
 Chloromethylhalicyclamine B, a Marine-Derived Protein Kinase CK1δ/ε Inhibitor Germana Esposito, Marie-Lise Bourguet-Kondracki, * Linh H. Mai, Arlette Longeon, Roberta Teta, Laurent Meijer, Rob Van Soest,
Chloromethylhalicyclamine B, a Marine-Derived Protein Kinase CK1δ/ε Inhibitor Germana Esposito, Marie-Lise Bourguet-Kondracki, * Linh H. Mai, Arlette Longeon, Roberta Teta, Laurent Meijer, Rob Van Soest,
BIOXHMEIA, TOMOΣ I ΠANEΠIΣTHMIAKEΣ EKΔOΣEIΣ KPHTHΣ ΑΠΕΙΚΟΝΙΣΗ ΜΟΡΙΑΚΩΝ ΔΟΜΩΝ
 BIOXHMEIA, TOMOΣ I ΠANEΠIΣTHMIAKEΣ EKΔOΣEIΣ KPHTHΣ ΑΠΕΙΚΟΝΙΣΗ ΜΟΡΙΑΚΩΝ ΔΟΜΩΝ ΑΠΕΙΚΟΝΙΣΗ ΜΟΡΙΑΚΩΝ ΔΟΜΩΝ ΜΟΡΙΑΚΑ ΜΟΝΤΕΛΑ 1: ΧΩΡΟΠΛΗΡΩΤΙΚΟ ΜΟΝΤΕΛΟ (SPACE-FILLING) 1: ΧΩΡΟΠΛΗΡΩΤΙΚΟ ΜΟΝΤΕΛΟ (SPACE-FILLING)
BIOXHMEIA, TOMOΣ I ΠANEΠIΣTHMIAKEΣ EKΔOΣEIΣ KPHTHΣ ΑΠΕΙΚΟΝΙΣΗ ΜΟΡΙΑΚΩΝ ΔΟΜΩΝ ΑΠΕΙΚΟΝΙΣΗ ΜΟΡΙΑΚΩΝ ΔΟΜΩΝ ΜΟΡΙΑΚΑ ΜΟΝΤΕΛΑ 1: ΧΩΡΟΠΛΗΡΩΤΙΚΟ ΜΟΝΤΕΛΟ (SPACE-FILLING) 1: ΧΩΡΟΠΛΗΡΩΤΙΚΟ ΜΟΝΤΕΛΟ (SPACE-FILLING)
Ποια είναι κατά τη γνώμη σας τα 30 μικρομόρια που συνιστούν τα πρόδρομα μόρια των βιομακρομορίων; Πώς μπορούν να ταξινομηθούν;
 Ποια είναι κατά τη γνώμη σας τα 30 μικρομόρια που συνιστούν τα πρόδρομα μόρια των βιομακρομορίων; Πώς μπορούν να ταξινομηθούν; Γενικά Για να προσδιορίσουμε τα 30 πρόδρομα μόρια των βιομακρομορίων θα πρέπει
Ποια είναι κατά τη γνώμη σας τα 30 μικρομόρια που συνιστούν τα πρόδρομα μόρια των βιομακρομορίων; Πώς μπορούν να ταξινομηθούν; Γενικά Για να προσδιορίσουμε τα 30 πρόδρομα μόρια των βιομακρομορίων θα πρέπει
Supplementary Materials: Development of Amyloseand β-cyclodextrin-based Chiral Fluorescent Sensors Bearing Terthienyl Pendants
 S1 of S12 Supplementary Materials: Development of Amyloseand β-cyclodextrin-based Chiral Fluorescent Sensors Bearing Terthienyl Pendants Tomoyuki Ikai, Changsik Yun, Yutaka Kojima, Daisuke Suzuki, Katsuhiro
S1 of S12 Supplementary Materials: Development of Amyloseand β-cyclodextrin-based Chiral Fluorescent Sensors Bearing Terthienyl Pendants Tomoyuki Ikai, Changsik Yun, Yutaka Kojima, Daisuke Suzuki, Katsuhiro
Cyclic Cystine-Bridged Peptides from the Marine. Sponge Clathria basilana Induce Apoptosis in. Tumor Cells and Depolarize the Bacterial
 Supporting information Cyclic Cystine-Bridged Peptides from the Marine Sponge Clathria basilana Induce Apoptosis in Tumor Cells and Depolarize the Bacterial Cytoplasmic Membrane Amin Mokhlesi,, Fabian
Supporting information Cyclic Cystine-Bridged Peptides from the Marine Sponge Clathria basilana Induce Apoptosis in Tumor Cells and Depolarize the Bacterial Cytoplasmic Membrane Amin Mokhlesi,, Fabian
Rh(III)-Catalyzed C-H Amidation with N-hydroxycarbamates: A. new Entry to N-Carbamate Protected Arylamines
 Rh(III)-Catalyzed C-H Amidation with N-hydroxycarbamates: A new Entry to N-Carbamate Protected Arylamines Bing Zhou,* Juanjuan Du, Yaxi Yang,* Huijin Feng, Yuanchao Li Shanghai Institute of Materia Medica,
Rh(III)-Catalyzed C-H Amidation with N-hydroxycarbamates: A new Entry to N-Carbamate Protected Arylamines Bing Zhou,* Juanjuan Du, Yaxi Yang,* Huijin Feng, Yuanchao Li Shanghai Institute of Materia Medica,
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Heronamides A C, new polyketide macrolactams from an Australian marine-derived Streptomyces sp. A biosynthetic case for synchronized tandem electrocyclization. Ritesh Raju, Andrew
SUPPORTING INFORMATION Heronamides A C, new polyketide macrolactams from an Australian marine-derived Streptomyces sp. A biosynthetic case for synchronized tandem electrocyclization. Ritesh Raju, Andrew
Free Radical Initiated Coupling Reaction of Alcohols and. Alkynes: not C-O but C-C Bond Formation. Context. General information 2. Typical procedure 2
 Free Radical Initiated Coupling Reaction of Alcohols and Alkynes: not C-O but C-C Bond Formation Zhongquan Liu,* Liang Sun, Jianguo Wang, Jie Han, Yankai Zhao, Bo Zhou Institute of Organic Chemistry, Gannan
Free Radical Initiated Coupling Reaction of Alcohols and Alkynes: not C-O but C-C Bond Formation Zhongquan Liu,* Liang Sun, Jianguo Wang, Jie Han, Yankai Zhao, Bo Zhou Institute of Organic Chemistry, Gannan
Composition Analysis of Protein and Oil and Amino Acids of the Soybean Varieties in Heilongjiang Province of China
 29 4 2003 7 551 556 ACTA AGRONOMICA SINICA Vol. 29, No. 4 pp. 551 556 July, 2003 (, 150030) 62,, ;,, 19 3, 9 6, Ξ, ; ; ; : S565 : A Composition Analysis of Protein and Oil and Amino Acids of the Soybean
29 4 2003 7 551 556 ACTA AGRONOMICA SINICA Vol. 29, No. 4 pp. 551 556 July, 2003 (, 150030) 62,, ;,, 19 3, 9 6, Ξ, ; ; ; : S565 : A Composition Analysis of Protein and Oil and Amino Acids of the Soybean
Available online at
 J. Serb. Chem. Soc. 76 (12) S1 S5 (2011) Supplementary material SUPPLEMENTARY MATERIAL TO Synthesis of quinoline-attached furan-2(3h)-ones having anti-inflammatory and antibacterial properties with reduced
J. Serb. Chem. Soc. 76 (12) S1 S5 (2011) Supplementary material SUPPLEMENTARY MATERIAL TO Synthesis of quinoline-attached furan-2(3h)-ones having anti-inflammatory and antibacterial properties with reduced
Supplementary information:
 Supplementary information: Monitoring changes of docosahexaenoic acid-containing lipids during the recovery process of traumatic brain injury in rat using mass spectrometry imaging Shuai Guo 1, Dan Zhou
Supplementary information: Monitoring changes of docosahexaenoic acid-containing lipids during the recovery process of traumatic brain injury in rat using mass spectrometry imaging Shuai Guo 1, Dan Zhou
Copper-mediated radical cross-coupling reaction of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) with phenols or thiophenols. Support Information
 Copper-mediated radical cross-coupling reaction of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) with phenols or thiophenols Dr. Xiao un Tang and Prof. Qing un Chen* Key Laboratory of Organofluorine Chemistry,
Copper-mediated radical cross-coupling reaction of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123) with phenols or thiophenols Dr. Xiao un Tang and Prof. Qing un Chen* Key Laboratory of Organofluorine Chemistry,
Supporting Information
 Supporting Information Ceric Ammonium Nitrate (CAN) catalyzed efficient one-pot three component aza-diels-alder reactions for a facile synthesis of tetrahydropyranoquinoline derivatives Ravinder Goud Puligoundla
Supporting Information Ceric Ammonium Nitrate (CAN) catalyzed efficient one-pot three component aza-diels-alder reactions for a facile synthesis of tetrahydropyranoquinoline derivatives Ravinder Goud Puligoundla
Supplementary Information
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Supplementary Information A contribution to the rational design of Ru(CO) 3 Cl 2 L complexes
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Supplementary Information A contribution to the rational design of Ru(CO) 3 Cl 2 L complexes
Chemical Constituents and Antioxidant Activity of Teucrium barbeyanum Aschers.
 Supporting Information Rec. Nat. Prod. 9:1 (2015) 159-163 Chemical Constituents and Antioxidant Activity of Teucrium barbeyanum Aschers. Mohamed Ali A. Alwahsh, Melati Khairuddean * and Wong Keng Chong
Supporting Information Rec. Nat. Prod. 9:1 (2015) 159-163 Chemical Constituents and Antioxidant Activity of Teucrium barbeyanum Aschers. Mohamed Ali A. Alwahsh, Melati Khairuddean * and Wong Keng Chong
Study on anti-hyperlipidemia mechanism of high frequency herb pairs by molecular docking method
 2015 6 40 12 Vol. 40 No. 12 June 2015 *,,,, (, 100102) 2010 3 - - - 20 3- -3- A HMG-CoA reductase α PPAR-α Niemann-Pick C1 like 1 NPC1L1 3 LibDock RMSD 3 A 4 2 5 Study on anti-hyperlipidemia mechanism
2015 6 40 12 Vol. 40 No. 12 June 2015 *,,,, (, 100102) 2010 3 - - - 20 3- -3- A HMG-CoA reductase α PPAR-α Niemann-Pick C1 like 1 NPC1L1 3 LibDock RMSD 3 A 4 2 5 Study on anti-hyperlipidemia mechanism
Lewis Acid Catalyzed Propargylation of Arenes with O-Propargyl Trichloroacetimidate: Synthesis of 1,3-Diarylpropynes
 Supporting Information for Lewis Acid Catalyzed Propargylation of Arenes with O-Propargyl Trichloroacetimidate: Synthesis of 1,3-Diarylpropynes Changkun Li and Jianbo Wang* Beijing National Laboratory
Supporting Information for Lewis Acid Catalyzed Propargylation of Arenes with O-Propargyl Trichloroacetimidate: Synthesis of 1,3-Diarylpropynes Changkun Li and Jianbo Wang* Beijing National Laboratory
MAΘΗΜΑ 4 ο AMINOΞΕΑ-ΠΕΠΤΙ ΙΑ-ΠΡΩΤΕΪΝΕΣ
 MAΘΗΜΑ 4 ο AMIΞΕΑ-ΠΕΠΤΙ ΙΑ-ΠΡΩΤΕΪΝΕΣ Αλανίνη (Αla) Αλανυλοσερίνη (Αla-Ser) Αλβουµίνη ρα. Κουκουλίτσα Αικατερίνη Χηµικός Εργαστηριακός Συνεργάτης Τ.Ε.Ι Αθήνας ckoukoul@teiath.gr AMIΞΕΑ 2 λειτουργικές οµάδες
MAΘΗΜΑ 4 ο AMIΞΕΑ-ΠΕΠΤΙ ΙΑ-ΠΡΩΤΕΪΝΕΣ Αλανίνη (Αla) Αλανυλοσερίνη (Αla-Ser) Αλβουµίνη ρα. Κουκουλίτσα Αικατερίνη Χηµικός Εργαστηριακός Συνεργάτης Τ.Ε.Ι Αθήνας ckoukoul@teiath.gr AMIΞΕΑ 2 λειτουργικές οµάδες
Electronic Supplementary Information:
 Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Electronic Supplementary Information: 2 6 5 7 2 N S 3 9 6 7 5 2 S N 3 9 Scheme S. Atom numbering for thione and thiol tautomers of thioacetamide 3 0.6 0. absorbance 0.2 0.0 0.5 0.0 0.05 0.00 N 2 Ar km/mol
Supporting Information. Introduction of a α,β-unsaturated carbonyl conjugated pyrene-lactose hybrid
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 16 Supporting Information Introduction of a α,β-unsaturated carbonyl conjugated pyrene-lactose hybrid
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 16 Supporting Information Introduction of a α,β-unsaturated carbonyl conjugated pyrene-lactose hybrid
Supporting Information. Palladium Complexes with Bulky Diphosphine. Synthesis of (Bio-) Adipic Acid from Pentenoic. Acid Mixtures.
 Supporting Information Palladium Complexes with Bulky Diphosphine Ligands as Highly Selective Catalysts for the Synthesis of (Bio-) Adipic Acid from Pentenoic Acid Mixtures. Choon Heng Low, James D. Nobbs,*
Supporting Information Palladium Complexes with Bulky Diphosphine Ligands as Highly Selective Catalysts for the Synthesis of (Bio-) Adipic Acid from Pentenoic Acid Mixtures. Choon Heng Low, James D. Nobbs,*
multicomponent synthesis of 5-amino-4-
 Supporting Informartion for Molecular iodine-catalyzed one-pot multicomponent synthesis of 5-amino-4- (arylselanyl)-1h-pyrazoles Camila S. Pires 1, Daniela H. de Oliveira 1, Maria R. B. Pontel 1, Jean
Supporting Informartion for Molecular iodine-catalyzed one-pot multicomponent synthesis of 5-amino-4- (arylselanyl)-1h-pyrazoles Camila S. Pires 1, Daniela H. de Oliveira 1, Maria R. B. Pontel 1, Jean
Iodine-catalyzed synthesis of sulfur-bridged enaminones and chromones via double C(sp 2 )-H thiolation
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Iodine-catalyzed synthesis of sulfur-bridged enaminones and chromones via
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2017 Iodine-catalyzed synthesis of sulfur-bridged enaminones and chromones via
ΤΕΛΟΣ 1ΗΣ ΑΠΟ 6 ΣΕΛΙΔΕΣ
 ΘΕΜΑ Α ΑΡΧΗ 1ΗΣ ΣΕΛΙΔΑΣ Δ ΕΣΠΕΡΙΝΩΝ ΠΑΝΕΛΛΑΔΙΚΕΣ ΕΞΕΤΑΣΕΙΣ Δ ΤΑΞΗΣ ΕΣΠΕΡΙΝΟΥ ΓΕΝΙΚΟΥ ΛΥΚΕΙΟΥ ΤΡΙΤΗ 19 ΙΟΥΝΙΟΥ 2018 ΕΞΕΤΑΖΟΜΕΝΟ ΜΑΘΗΜΑ: ΒΙΟΛΟΓΙΑ ΠΡΟΣΑΝΑΤΟΛΙΣΜΟΥ ΣΥΝΟΛΟ ΣΕΛΙΔΩΝ: ΕΞΙ (6) Να γράψετε στο τετράδιό
ΘΕΜΑ Α ΑΡΧΗ 1ΗΣ ΣΕΛΙΔΑΣ Δ ΕΣΠΕΡΙΝΩΝ ΠΑΝΕΛΛΑΔΙΚΕΣ ΕΞΕΤΑΣΕΙΣ Δ ΤΑΞΗΣ ΕΣΠΕΡΙΝΟΥ ΓΕΝΙΚΟΥ ΛΥΚΕΙΟΥ ΤΡΙΤΗ 19 ΙΟΥΝΙΟΥ 2018 ΕΞΕΤΑΖΟΜΕΝΟ ΜΑΘΗΜΑ: ΒΙΟΛΟΓΙΑ ΠΡΟΣΑΝΑΤΟΛΙΣΜΟΥ ΣΥΝΟΛΟ ΣΕΛΙΔΩΝ: ΕΞΙ (6) Να γράψετε στο τετράδιό
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Synthesis of 3-omosubstituted Pyrroles via Palladium- Catalyzed Intermolecular Oxidative Cyclization
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Synthesis of 3-omosubstituted Pyrroles via Palladium- Catalyzed Intermolecular Oxidative Cyclization
Electronic Supplementary information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary information Sulfonic acid-functionalized MIL-101(Cr) as a highly efficient
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary information Sulfonic acid-functionalized MIL-101(Cr) as a highly efficient
Supporting Information for Iron-catalyzed decarboxylative alkenylation of cycloalkanes with arylvinylic carboxylic acids via a radical process
 Supporting Information for Iron-catalyzed decarboxylative alkenylation of cycloalkanes with arylvinylic carboxylic acids via a radical process Jincan Zhao 1, Hong Fang 1, Jianlin Han* 1,2 and Yi Pan* 1
Supporting Information for Iron-catalyzed decarboxylative alkenylation of cycloalkanes with arylvinylic carboxylic acids via a radical process Jincan Zhao 1, Hong Fang 1, Jianlin Han* 1,2 and Yi Pan* 1
The Free Internet Journal for Organic Chemistry
 The Free Internet Journal for Organic Chemistry Paper Archive for Organic Chemistry Arkivoc 2018, part iii, S1-S6 Synthesis of dihydropyranones and dihydropyrano[2,3- d][1,3]dioxine-diones by cyclization
The Free Internet Journal for Organic Chemistry Paper Archive for Organic Chemistry Arkivoc 2018, part iii, S1-S6 Synthesis of dihydropyranones and dihydropyrano[2,3- d][1,3]dioxine-diones by cyclization
Κεφάλαιο 2ο. Αντιγραφή, έκφραση και ρύθμιση της γενετικής πληροφορίας
 Κεφάλαιο 2ο Αντιγραφή, έκφραση και ρύθμιση της γενετικής πληροφορίας 1. Το DNA αυτοδιπλασιάζεται 3ο ε.λ. Ηλιούπολης επιμέλεια: Αργύρης Γιάννης 3 Ο μηχανισμός της αντιγραφής του DNA Ο μηχανισμός αυτοδιπλασιασμού
Κεφάλαιο 2ο Αντιγραφή, έκφραση και ρύθμιση της γενετικής πληροφορίας 1. Το DNA αυτοδιπλασιάζεται 3ο ε.λ. Ηλιούπολης επιμέλεια: Αργύρης Γιάννης 3 Ο μηχανισμός της αντιγραφής του DNA Ο μηχανισμός αυτοδιπλασιασμού
Heterobimetallic Pd-Sn Catalysis: Michael Addition. Reaction with C-, N-, O-, S- Nucleophiles and In-situ. Diagnostics
 Supporting Information (SI) Heterobimetallic Pd-Sn Catalysis: Michael Addition Reaction with C-, N-, -, S- Nucleophiles and In-situ Diagnostics Debjit Das, a Sanjay Pratihar a,b and Sujit Roy c * a rganometallics
Supporting Information (SI) Heterobimetallic Pd-Sn Catalysis: Michael Addition Reaction with C-, N-, -, S- Nucleophiles and In-situ Diagnostics Debjit Das, a Sanjay Pratihar a,b and Sujit Roy c * a rganometallics
Supplementary Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 215 Supplementary Information Zinc monoglycerolate as a catalyst for the conversion of 1,3- and
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 215 Supplementary Information Zinc monoglycerolate as a catalyst for the conversion of 1,3- and
Regioselectivity in the Stille coupling reactions of 3,5- dibromo-2-pyrone.
 Regioselectivity in the Stille coupling reactions of 3,5- dibromo-2-pyrone. Won-Suk Kim, Hyung-Jin Kim and Cheon-Gyu Cho Department of Chemistry, Hanyang University, Seoul 133-791, Korea Experimental Section
Regioselectivity in the Stille coupling reactions of 3,5- dibromo-2-pyrone. Won-Suk Kim, Hyung-Jin Kim and Cheon-Gyu Cho Department of Chemistry, Hanyang University, Seoul 133-791, Korea Experimental Section
