Thermofluids Data Book for Part I of the Engineering Tripos
|
|
|
- Αμύντας Βάμβας
- 8 χρόνια πριν
- Προβολές:
Transcript
1 Thermofluids Data Book for Part I of the Engineering Tripos 004 Edition Cambridge University Engineering Department
2 CONTENTS Thermodynamic definitions & relationships... Ideal gas relationships... 3 Perfect gas relationships... 3 Mixtures of perfect gases... 3 Non-dimensional groups... 4 Heat transfer... 5 Equations for systems... 6 Equations for control volumes... 7 Equations for streamlines... 9 Incompressible viscous pipe flow... 9 Equations of motion in differential form for viscous steady flow of a fluid... 9 Thermodynamic efficiencies... 0 Combustion... Properties of perfect gases... Molar enthalpies of common gases at low pressures... 3 Thermochemical data for equilibrium reactions... 4 Data for steam... 6 Triple point data for steam... 6 Critical point data for steam... 6 Properties of saturated water & steam: Temperatures from the triple point to the critical point. 7 Properties of saturated water & steam: Pressures from the triple point to the critical point... 9 Specific enthalpy of water and steam...3 Specific Entropy of water and steam... 4 Density of water and steam... 5 Specific internal energy of water and steam... 6 Transport properties of saturated water & steam... 7 Transport properties of steam... 8 Transport properties of air... 8 Transport properties of carbon dioxide... 9 Transport properties of hydrogen... 9 Perfect gas relations for compressible flow for γ= Properties of gases at sea level conditions... 3 Properties of liquids at sea level conditions... 3 The International Standard Atmosphere... 3 Properties of the International Standard Atmosphere at altitude... 3 Physical constants Conversion of Non-SI to SI Units Conversion of Non-SI to SI Units cont Sources of Information Critical point data for Refrigerant R-34a (CH FCF 3 ) Properties Table for Refrigerant R-34a (CH FCF 3 ) Properties Chart for Refrigerant R-34a (CH FCF 3 ) Properties of steam /09/04
3 THERMODYNAMIC DEFINITIONS & RELATIONSHIPS Specific enthalpy Specific heat capacity at constant volume Specific heat capacity at constant pressure Ratio of specific heat capacities Coefficient of volume expansion Coefficient of compressibility For a simple compressible substance, in the absence of capillarity, electric and magnetic fields h u + c c v p γ pv u T v h T c c p v p v β v T p v κ v p T Tds = du + pdv = dh vdp 06/09/04
4 IDEAL GAS RELATIONSHIPS Equation of state Relationship between c p, c v and R pv = nrt pv = mrt pv = RT p = ρrt c p cv = R Speed of sound a = γrt PERFECT GAS RELATIONSHIPS Change in specific internal energy u u = cv ( T ) T Change in specific enthalpy h h = c p ( T ) T Change in specific entropy For Isentropic changes s T v c v ln +Rln T v T p s = c p ln Rln T p p ln v c +c ln v p p v γ pv = const. γ Tv = const. ( γ T / p ) γ = const. MIXTURES OF PERFECT GASES For a mixture of N perfect gases where, for component -i, m i = mass, p i = partial pressure, h i = h i (T) = partial specific enthalpy, s i = s i (T, p i ) = partial specific entropy, n i = number of mols and the overbar signifies a partial molar quantity: Pressure of the mixture = p mixture = p i i= i N Enthalpy of the mixture Entropy of the mixture H S mixture mixture = = i = N i= i= N i= m h i m s i i i = = i= N n h i i= i = N n s i i= i i 06/09/04 3
5 Reynolds Number Mach Number Froude Number Prandtl Number Drag Coefficient Lift Coefficient NON-DIMENSIONAL GROUPS ρvd Vd Re = = µ ν V M = a Fr = V gz µ c p Pr = = λ = D C D ρ V = L C L ρ V τ Skin Friction Coefficient c w f = V ρ Friction Factor Discharge Coefficient Nusselt Number Grashof Number Stanton Number f = 4c f C d m = m hd Nu = λ actual ideal 3 ν α A A gd β T Gr = ν St = Nu RePr h = ρvc p 06/09/04 4
6 HEAT TRANSFER Conduction, Convection and Radiation Rate of heat transfer Q by convection from a body of surface area A ( ) Q = ha T body T surroundings Rate of heat transfer Q by conduction along a straight bar of cross-sectional area A dt Q = λa dx Rate of heat transfer Q by conduction radially in a straight circular bar of length L dt Q = λπrl dr Rate of heat transfer Q by radiation from a grey body of surface area A and emissivity ε 4 Q = ε Q black = εσat where σ = 5.67x0-8 Wm - K -4 is the Stefan-Boltzmann constant. Logarithmic-Mean Temperature Difference (LMTD) T m T = ln T ( T T ) Convective heat transfer for fully developed flow in circular pipes of diameter d Overall heat transfer for laminar flow with constant wall temperature Nu = 3.66 Re < 300 d Overall heat transfer for turbulent flow with constant wall temperature Nu d = 0.8 d 0.03Re Pr 0.4 d 7 0. < Pr 300 Re <0 0.5 < Pr < 0 < d Heat transfer due to natural convection from a vertical isothermal plate of height L Overall heat transfer ( ) 0. 5 Nu L = 0.5 GrPr Pr Reynolds Analogy c f St = Note that the net rate of heat transfer will be less due to incident radiation 06/09/04 5
7 Definition of a system A system is a fixed quantity of matter. Conservation of mass applied to a system The mass of a system is constant, i.e. EQUATIONS FOR SYSTEMS m = const. st Law of Thermodynamics applied to a system When heat transfer to, and work done by, a system are defined as positive and the system is undergoing a cyclic process dq = dw When heat transfer to, and work done by, a system of mass m undergoing a process between state and state are defined as positive and in the absence of capillarity, electric and magnetic fields Q W = E E = ( U + mv + mgz ) ( U + mv + mgz ) nd Law of Thermodynamics applied to a system When heat transfer to a system undergoing a cyclic process is defined as positive, the Clausius inequality is dq 0 T When heat transfer to a system of mass m undergoing a process is defined as positive dq mds = + mds irrev where dsirrev T where ds is the entropy created per unit mass by irreversibilities. irrev pdv work for a system When the surface of a system undergoes displacement between state and state and the pressure causing the displacement is known over the entire surface that is displaced, the displacement work done by the system during this process is W = pdv 0 06/09/04 6
8 EQUATIONS FOR CONTROL VOLUMES Definition of a control volume A control volume is that region of space that is enclosed by a rigid control surface. The requirements for steady flow within a control volume For steady-flow, conditions within the control volume are not, on average, changing so that the mass, momentum, energy and entropy within the control volume remain constant. Conservation of mass applied to a control volume - the Continuity Equation In general, this may be written in vector form as d dt cv ρdv + cs ρv da = 0 where da is positive out of the control volume. It may also be written as dmcv + m out m in = 0 dt For steady flow, the above becomes m out = m in Momentum equations applied to a control volume, including the Steady Flow Momentum Equation (SFME) In general, this may be written in vector form as d dt cv ρvdv + cs ρvv da = F where da is positive out of the control volume and F is the sum of all the non-pressure forces on the flow. cs pda For steady flow, the above becomes the Steady Flow Momentum Equation (SFME) and in x and y coordinates, the SFME is m m out out ρvv da = F cs V V cs x, out m invx, in y, out m inv y, in = = pda F F x y ( pa) x ( pa) y where care is needed with respect to the sign of the V x, V y and A terms. 06/09/04 7
9 st Law of Thermodynamics applied to a control volume, including the Steady Flow Energy Equation (SFEE) In the absence of capillarity, electric and magnetic fields, the st Law becomes ( h + V + gz ) m ( h + V + gz ) = Q W x decv + m out out dt out out in in in in where E cv is the total energy within the control volume, Q is the rate of heat transfer to the control volume and W x is the rate of shaft work transferred from the control volume. For steady flow, the above becomes the Steady Flow Energy Equation (SFEE) m out ( hout + Vout + gzout ) m in ( hin + Vin + gzin ) = Q W x nd Law of Thermodynamics applied to a control volume When heat transfer to a control volume is defined as positive ds dt cv dq + mout sout minsin S = + irrev where S irrev 0 T A where dq is the rate of heat transfer to an area da of the control surface at temperature T and the integration is over the whole area A of the control surface, S cv is the total entropy within the control volume and S irrev is the rate of entropy creation within the control volume due to irreversibilities. 06/09/04 8
10 EQUATIONS FOR STREAMLINES Bernoulli's Equation for incompressible inviscid steady flow along a streamline p + ρv + ρgz = const. The pressure gradient normal to a streamline with a radius of curvature R V dp dn ρv = R n,e n e s s R INCOMPRESSIBLE VISCOUS PIPE FLOW The pressure drop along a pipe of constant diameter d and length L, with viscous flow L L p = 4c f ρ V = f ρv d d EQUATIONS OF MOTION IN DIFFERENTIAL FORM FOR VISCOUS STEADY FLOW OF A FLUID Mass conservation (continuity) n Intrinsic coordinates. ( ) = 0 e s + e s Vector Notation. ( ρv ) = 0 n V e s Momentum Intrinsic coordinates Vector Notation V V p p ρ V es e n = es en + ρ g + viscous s R s n ρv V = p + ρ g + viscous 06/09/04 9
11 THERMODYNAMIC EFFICIENCIES Efficiency of a cycle W ηcycle Q but so W η net cycle = Q B net B Q Q = Q A B A Isentropic efficiencies of compressors and turbines. Q B. Q A. W Net η c Ideal Work Input Actual Work Input = h s h h h η t Actual Work Output Ideal Work Output h = h 3 3 h h 4 4s 06/09/04 0
12 COMBUSTION The SFEE applied to stoichiometric combustion at constant T and p When heat transfer to a control volume is defined as positive and in the absence of shaft work, changes in KE and PE, capillarity, electric and magnetic fields, the rate of heat transfer and the calorific value (CV ) are related by where ( CV ) n h Q = m = fuel m = M fuel n fuel Calorific values of common fuels (Enthalpies of Reaction) In the following table, the calorific value is equal and opposite to the enthalpy of reaction when the reactants and products are at 5 C and.034 bar. In the evaluation of the lower calorific value and lower enthalpy of reaction, the steam is assumed to be dry saturated. Stoichiometric Equation C Molar Mass M of Fuel kg kmol fuel Phase Calorific Value MJ/kg O CO solid C + O CO solid CO + O CO 8 gas 0.00 Higher: H O Lower: H O to water to steam + O H O gas H CH4 + O CO + HO 6 gas H + 3.5O CO + H O 30 gas C 6 3 C3H8 + 5 O 3CO + 4H O 44 gas H + 6.5O 4CO + H O 58 gas C4 0 5 C8H8 +.5O 8CO + 9H O 4 gas H +.5O 8CO + H O 4 liquid C8 8 9 A more exact value is.06 06/09/04
13 PROPERTIES OF PERFECT GASES Values of M, R, c p, c v and γ At normal atmospheric conditions, and over a limited range of temperature and pressure, the gases listed below may be assumed to behave as perfect gases. That is, they may be assumed to have the equation of state pv = RT, and to have constant specific heat capacities. Gas Molar mass M kg/kmol Gas constant R kj/kg K c p kj/kg K c v kj/kg K Air # Atmospheric nitrogen N O A H * He CO CO SO CH C H C 3 H Real gases are not perfect gases, and the rounded values for R, c p c v and c p cv listed above do not exactly satisfy the relationships between these quantities that would be obtained for perfect gases. γ c c p v Molar (universal) gas constant R = MR = kj/kmol K Molar volume of a perfect gas kmol of any perfect gas occupies a volume of approximately.4 m 3 at s.t.p. (0 C and bar) and contains 6.0x0 6 particles. # Air contains.0% O and 79.0% atmospheric nitrogen by volume (Volumetric and Molar Analyses); 3.% O and 76.8% atmospheric nitrogen by weight (Gravimetric Analysis). Air contains 0.93 % of argon (A) and traces of other gases; these and the nitrogen together are called atmospheric nitrogen. * A more exact value is /09/04
14 MOLAR ENTHALPIES OF COMMON GASES AT LOW PRESSURES At low pressures, and over the temperature range quoted, the gases listed in this Table behave as semi-perfect gases. That is, while having the molar equation of state pv = RT, their specific heat capacities c p and c v are not constant but are functions only of temperature. Gas Air N O H CO CO H O Gas Molar Mass kg/kmol Molar Mass kg/kmol Temperature K Molar enthalpy MJ/kmol Temperature K =5 C C= Notes: () The molar enthalpies listed are those in the ideal gas state at zero pressure, but the values given are also valid at and around atmospheric pressure. () In this table, the arbitrary datum state for zero enthalpy is that of the substance in the ideal gas state at zero pressure and zero absolute temperature. (3) The values for atmospheric nitrogen, * N, may be taken to be the same as those for N. A more exact value is.06 06/09/04 3
15 THERMOCHEMICAL DATA FOR EQUILIBRIUM REACTIONS The tables of equilibrium constants and standard enthalpy change on the next page relate to the reactions listed below Stoichiometric equations vi A i = 0 i where v i is the stoichiometric coefficient of the substance whose chemical symbol is A i. () H + H = 0 (4) NO + N + O = 0 (7) CO ½ O + CO = 0 () N + N = 0 (5) H ½ O + H O = 0 (8) CO H O + CO + H = 0 (3) O + O = 0 (6) ½ H OH + H O = 0 (9) ½ N 3 H + NH 3 = 0 Equilibrium constants The equilibrium constant * i K p is given by ln ( K p ) = where p pi p0 pi partial pressure of species Ai in bars p standard pressure bar 0 i i * i v ln p Thus * p i is numerically equal to p i but is dimensionless. Standard free enthalpy of reaction At a given temperature, the standard free enthalpy of reaction (or the standard Gibbs function change) ln by the following equation: 0 G T may be calculated from the listed value of ( ) K p G 0 T = RT ln K p = T ln( K ) kj p Standard enthalpy of reaction The Standard enthalpy of reaction is given by where 0 ( f T ) i where [ ] H 0 0 [ h ] = ν ([ H ] ) ν i i T i 0 ~ T = i ~ [ hi ] T = ([ H f ] 98 ) + ([ h ] T [ h ] 98 ) i Η is the standard enthalpy of formation of species i at temperature T and a pressure p 0 = bar. i i f T i 06/09/04 4
16 Equilibrium constants & standard enthalpies of reaction Reaction Number v i = 0 ½ ½ ½ 0 i Temperature Equilibrium Constant ln( ) K Temperature K K p Standard Enthalpy of Reaction MJ 0 H T Warning: These tables list absolute temperatures 06/09/04 5
17 DATA FOR STEAM Source of data The follow ing tables have been produced using equations from the IAPW S Form ulation 995 for the Therm odynam ic Properties ofordinary W ater Substance for G eneraland Scientific Use and the Supplem entary R elease: Saturation Properties of Ordinary W ater Substance. These docum ents can be found on the InternationalA ssociation for the Properties of W ater and Steam w ebsite w w.iapw s.org. TRIPLE POINT DATA FOR STEAM Temperature = 73.6 K (0.0 o C) Pressure = bar Phase Specific volume Specific enthalpy Specific entropy m 3 /kg kj/kg kj/kg K Ice Water Steam CRITICAL POINT DATA FOR STEAM Temperature = K ( C) Pressure = 0.64 bar Density = 3 kg/m 3 06/09/04 6
18 PROPERTIES OF SATURATED WATER & STEAM: Temperatures from the triple point to the critical point Temp. Pressure Specific volume Spec. int. energy Specific enthalpy Specific entropy Temp. o C bar m 3/ kg kj/kg kj/kg kj/kg K o C T p v f v g u f u g h f h fg h g s f s g T T p v f v g u f u g h f h fg h g s f s g T 06/09/04 7
19 Properties of Saturated Water & Steam continued: Temperatures from the triple point to the critical point Temp. Pressure Specific volume Spec. int. energy Specific enthalpy Specific entropy Temp. o C bar m 3/ kg kj/kg kj/kg kj/kg K o C T p v f v g u f u g h f h fg h g s f s g T T p v f v g u f u g h f h fg h g s f s g T 06/09/04 8
20 PROPERTIES OF SATURATED WATER & STEAM: Pressures from the triple point to the critical point Pressure Temp. Specific volume Spec. int. energy Specific enthalpy Specific entropy Pressure bar o C m 3/ kg kj/kg kj/kg kj/kg K bar p T v f v g u f u g h f h fg h g s f s g p p T v f v g u f u g h f h fg h g s f s g p 06/09/04 9
21 Properties of Saturated Water & Steam continued: Pressures from the triple point to the critical point Pressure Temp. Specific volume Spec. int. energy Specific enthalpy Specific entropy Pressure bar o C m 3/ kg kj/kg kj/kg kj/kg K bar p T v f v g u f u g h f h fg h g s f s g p p T v f v g u f u g h f h fg h g s f s g p 06/09/04 0
22 Properties of Saturated Water & Steam continued: Pressures from the triple point to the critical point Pressure Temp. Specific volume Spec. int. energy Specific enthalpy Specific entropy Pressure bar o C m 3/ kg kj/kg kj/kg kj/kg K bar p T v f v g u f u g h f h fg h g s f s g p p T v f v g u f u g h f h fg h g s f s g p 06/09/04
23 Properties of Saturated Water & Steam continued: Pressures from the triple point to the critical point Pressure Temp. Specific volume Spec. int. energy Specific enthalpy Specific entropy Pressure bar o C m 3/ kg kj/kg kj/kg kj/kg K bar p T v f v g u f u g h f h fg h g s f s g p p T v f v g u f u g h f h fg h g s f s g p 06/09/04
24 Pressure (bar) SPECIFIC ENTHALPY OF WATER AND STEAM kj/kg Critical Isobar Temp ( o C) /09/04 3
25 Pressure (bar) SPECIFIC ENTROPY OF WATER AND STEAM kj/kg K Critical Isobar Temp ( o C) /09/04 4
26 Pressure (bar) DENSITY OF WATER AND STEAM kg/m Critical Isobar Temp ( o C) /09/04 5
27 Pressure (bar) SPECIFIC INTERNAL ENERGY OF WATER AND STEAM kj/kg Critical Isobar Temp ( o C) /09/04 6
28 Temp. C TRANSPORT PROPERTIES OF SATURATED WATER & STEAM Specific volume m 3 /kg v f vg Isobaric specific heat capacity kj/kg K c p f c pg Thermal conductivity W/m K λ f 3 λg µ f /0 Dynamic viscosity kg/s m 6 µ /0 g f Prandtl number = µ c p λ Pr Prg Temp. C 06/09/04 7
29 Temp. C TRANSPORT PROPERTIES OF STEAM Isobaric sp. heat capacity kj/kg K Thermal conductivity W/m K Dynamic viscosity kg/s m Prandtl number c λ 6 µ /0 Pr = µ c p λ p Values for water at atmospheric pressure between 0 C and 00 C are given with sufficient accuracy by the saturated values in the previous table. The above values are correct for a pressure of atm =.035 bar but may be used with sufficient accuracy at other pressures. Temp. C TRANSPORT PROPERTIES OF AIR Isobaric sp. heat capacity kj/kg K Thermal conductivity W/m K Dynamic viscosity kg/s m Prandtl number c λ 6 µ /0 Pr = µ c p λ p This table may be used with reasonable accuracy for values of c p, γ, µ and Pr of N, O and CO. The above values are correct for a pressure of atm =.035 bar but may be used with sufficient accuracy at other pressures. 06/09/04 8
DuPont Suva. DuPont. Thermodynamic Properties of. Refrigerant (R-410A) Technical Information. refrigerants T-410A ENG
 Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-410A ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 410A Refrigerant (R-410A) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
DuPont Suva 95 Refrigerant
 Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 ENG DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]
![Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)] Technical Information T-9100 SI. Suva. refrigerants. Thermodynamic Properties of. Suva Refrigerant [R-410A (50/50)]](/thumbs/89/98928029.jpg) d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
d Suva refrigerants Technical Information T-9100SI Thermodynamic Properties of Suva 9100 Refrigerant [R-410A (50/50)] Thermodynamic Properties of Suva 9100 Refrigerant SI Units New tables of the thermodynamic
DuPont Suva 95 Refrigerant
 Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Technical Information T-95 SI DuPont Suva refrigerants Thermodynamic Properties of DuPont Suva 95 Refrigerant (R-508B) The DuPont Oval Logo, The miracles of science, and Suva, are trademarks or registered
Mean bond enthalpy Standard enthalpy of formation Bond N H N N N N H O O O
 Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Q1. (a) Explain the meaning of the terms mean bond enthalpy and standard enthalpy of formation. Mean bond enthalpy... Standard enthalpy of formation... (5) (b) Some mean bond enthalpies are given below.
Figure 1 T / K Explain, in terms of molecules, why the first part of the graph in Figure 1 is a line that slopes up from the origin.
 Q1.(a) Figure 1 shows how the entropy of a molecular substance X varies with temperature. Figure 1 T / K (i) Explain, in terms of molecules, why the entropy is zero when the temperature is zero Kelvin.
Q1.(a) Figure 1 shows how the entropy of a molecular substance X varies with temperature. Figure 1 T / K (i) Explain, in terms of molecules, why the entropy is zero when the temperature is zero Kelvin.
STEAM TABLES. Mollier Diagram
 STEAM TABLES and Mollier Diagram (S.I. Units) dharm \M-therm\C-steam.pm5 CONTENTS Table No. Page No. 1. Saturated Water and Steam (Temperature) Tables I (ii) 2. Saturated Water and Steam (Pressure) Tables
STEAM TABLES and Mollier Diagram (S.I. Units) dharm \M-therm\C-steam.pm5 CONTENTS Table No. Page No. 1. Saturated Water and Steam (Temperature) Tables I (ii) 2. Saturated Water and Steam (Pressure) Tables
APPENDIX A. Summary of the English Engineering (EE) System of Units
 Appendixes A. Summary of the English Engineering (EE) System of Units B. Summary of the International System (SI) of Units C. Friction-Factor Chart D. Oblique-Shock Charts (γ = 1.4) (Two-Dimensional) E.
Appendixes A. Summary of the English Engineering (EE) System of Units B. Summary of the International System (SI) of Units C. Friction-Factor Chart D. Oblique-Shock Charts (γ = 1.4) (Two-Dimensional) E.
2. Chemical Thermodynamics and Energetics - I
 . Chemical Thermodynamics and Energetics - I 1. Given : Initial Volume ( = 5L dm 3 Final Volume (V = 10L dm 3 ext = 304 cm of Hg Work done W = ext V ext = 304 cm of Hg = 304 atm [... 76cm of Hg = 1 atm]
. Chemical Thermodynamics and Energetics - I 1. Given : Initial Volume ( = 5L dm 3 Final Volume (V = 10L dm 3 ext = 304 cm of Hg Work done W = ext V ext = 304 cm of Hg = 304 atm [... 76cm of Hg = 1 atm]
PROPERTIES OF ORDINARY WATER SUBSTANCE AT SATURATION FROF TME CRITICAL POINT DOWN TO 66 C EQUATIONS & TABLES
 PROPERTIES OF ORDINARY WATER SUBSTANCE AT SATURATION FROF TME CRITICAL POINT DOWN TO 66 C EQUATIONS & TABLES M. CONDE ENGINEERING, 2002 M. CONDE ENGINEERING, Zurich 2002 Properties of ordinary water substance
PROPERTIES OF ORDINARY WATER SUBSTANCE AT SATURATION FROF TME CRITICAL POINT DOWN TO 66 C EQUATIONS & TABLES M. CONDE ENGINEERING, 2002 M. CONDE ENGINEERING, Zurich 2002 Properties of ordinary water substance
CHAPTER 25 SOLVING EQUATIONS BY ITERATIVE METHODS
 CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
CHAPTER 5 SOLVING EQUATIONS BY ITERATIVE METHODS EXERCISE 104 Page 8 1. Find the positive root of the equation x + 3x 5 = 0, correct to 3 significant figures, using the method of bisection. Let f(x) =
Απόκριση σε Μοναδιαία Ωστική Δύναμη (Unit Impulse) Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο. Απόστολος Σ.
 Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο The time integral of a force is referred to as impulse, is determined by and is obtained from: Newton s 2 nd Law of motion states that the action
Απόκριση σε Δυνάμεις Αυθαίρετα Μεταβαλλόμενες με το Χρόνο The time integral of a force is referred to as impulse, is determined by and is obtained from: Newton s 2 nd Law of motion states that the action
Pof moist air and uses these properties to analyze conditions and
 CHAPTER 6 PSYCHROMETRICS Composition of Dry and Moist Air... 6.1 United States Standard Atmosphere... 6.1 Thermodynamic Properties of Moist Air... 6.2 Thermodynamic Properties of Water at Saturation...
CHAPTER 6 PSYCHROMETRICS Composition of Dry and Moist Air... 6.1 United States Standard Atmosphere... 6.1 Thermodynamic Properties of Moist Air... 6.2 Thermodynamic Properties of Water at Saturation...
Thermodynamic Tables to accompany Modern Engineering Thermodynamics
 Thermodynamic Tables to accompany Modern Engineering Thermodynamics This page intentionally left blank Thermodynamic Tables to accompany Modern Engineering Thermodynamics Robert T. Balmer AMSTERDAM BOSTON
Thermodynamic Tables to accompany Modern Engineering Thermodynamics This page intentionally left blank Thermodynamic Tables to accompany Modern Engineering Thermodynamics Robert T. Balmer AMSTERDAM BOSTON
Enthalpy data for the reacting species are given in the table below. The activation energy decreases when the temperature is increased.
 Q1.This question is about the reaction given below. O(g) + H 2O(g) O 2(g) + H 2(g) Enthalpy data for the reacting species are given in the table below. Substance O(g) H 2O(g) O 2(g) H 2(g) ΔH / kj mol
Q1.This question is about the reaction given below. O(g) + H 2O(g) O 2(g) + H 2(g) Enthalpy data for the reacting species are given in the table below. Substance O(g) H 2O(g) O 2(g) H 2(g) ΔH / kj mol
Jesse Maassen and Mark Lundstrom Purdue University November 25, 2013
 Notes on Average Scattering imes and Hall Factors Jesse Maassen and Mar Lundstrom Purdue University November 5, 13 I. Introduction 1 II. Solution of the BE 1 III. Exercises: Woring out average scattering
Notes on Average Scattering imes and Hall Factors Jesse Maassen and Mar Lundstrom Purdue University November 5, 13 I. Introduction 1 II. Solution of the BE 1 III. Exercises: Woring out average scattering
CH 3 CH 2 COOCH 2 CH 2 CH 3 + H 2 O
 Q1.An experiment was carried out to determine the equilibrium constant, K c, for the following reaction. CH 3 CH 2 COOH + CH 3 CH 2 CH 2 OH CH 3 CH 2 COOCH 2 CH 2 CH 3 + H 2 O A student added measured
Q1.An experiment was carried out to determine the equilibrium constant, K c, for the following reaction. CH 3 CH 2 COOH + CH 3 CH 2 CH 2 OH CH 3 CH 2 COOCH 2 CH 2 CH 3 + H 2 O A student added measured
Ύγρανση και Αφύγρανση. Ψυχρομετρία. 21-Nov-16
 Ύγρανση και Αφύγρανση Ψυχρομετρία η μελέτη των ιδιοτήτων του υγρού αέρα δηλαδή του μίγματος αέρα-ατμού-νερού. Ένα βασικό πρόβλημα: Δεδομένων των P (bar) Πίεση T ( o C) Θερμοκρασία Υ (kg/kg ξβ) Υγρασία
Ύγρανση και Αφύγρανση Ψυχρομετρία η μελέτη των ιδιοτήτων του υγρού αέρα δηλαδή του μίγματος αέρα-ατμού-νερού. Ένα βασικό πρόβλημα: Δεδομένων των P (bar) Πίεση T ( o C) Θερμοκρασία Υ (kg/kg ξβ) Υγρασία
By R.L. Snyder (Revised March 24, 2005)
 Humidity Conversion By R.L. Snyder (Revised March 24, 2005) This Web page provides the equations used to make humidity conversions and tables o saturation vapor pressure. For a pd ile o this document,
Humidity Conversion By R.L. Snyder (Revised March 24, 2005) This Web page provides the equations used to make humidity conversions and tables o saturation vapor pressure. For a pd ile o this document,
Chapter 22 - Heat Engines, Entropy, and the Second Law of Thermodynamics
 apter - Heat Engines, Entropy, and te Seond Law o ermodynamis.1 (a).0 J e 0.069 4 or 6.94% 60 J (b) 60 J.0 J J. e eat to melt 1.0 g o Hg is 4 ml 1 10 kg 1.18 10 J kg 177 J e energy absorbed to reeze 1.00
apter - Heat Engines, Entropy, and te Seond Law o ermodynamis.1 (a).0 J e 0.069 4 or 6.94% 60 J (b) 60 J.0 J J. e eat to melt 1.0 g o Hg is 4 ml 1 10 kg 1.18 10 J kg 177 J e energy absorbed to reeze 1.00
6.4 Superposition of Linear Plane Progressive Waves
 .0 - Marine Hydrodynamics, Spring 005 Lecture.0 - Marine Hydrodynamics Lecture 6.4 Superposition of Linear Plane Progressive Waves. Oblique Plane Waves z v k k k z v k = ( k, k z ) θ (Looking up the y-ais
.0 - Marine Hydrodynamics, Spring 005 Lecture.0 - Marine Hydrodynamics Lecture 6.4 Superposition of Linear Plane Progressive Waves. Oblique Plane Waves z v k k k z v k = ( k, k z ) θ (Looking up the y-ais
The kinetic and potential energies as T = 1 2. (m i η2 i k(η i+1 η i ) 2 ). (3) The Hooke s law F = Y ξ, (6) with a discrete analog
 Lecture 12: Introduction to Analytical Mechanics of Continuous Systems Lagrangian Density for Continuous Systems The kinetic and potential energies as T = 1 2 i η2 i (1 and V = 1 2 i+1 η i 2, i (2 where
Lecture 12: Introduction to Analytical Mechanics of Continuous Systems Lagrangian Density for Continuous Systems The kinetic and potential energies as T = 1 2 i η2 i (1 and V = 1 2 i+1 η i 2, i (2 where
Spherical Coordinates
 Spherical Coordinates MATH 311, Calculus III J. Robert Buchanan Department of Mathematics Fall 2011 Spherical Coordinates Another means of locating points in three-dimensional space is known as the spherical
Spherical Coordinates MATH 311, Calculus III J. Robert Buchanan Department of Mathematics Fall 2011 Spherical Coordinates Another means of locating points in three-dimensional space is known as the spherical
HOMEWORK 4 = G. In order to plot the stress versus the stretch we define a normalized stretch:
 HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
HOMEWORK 4 Problem a For the fast loading case, we want to derive the relationship between P zz and λ z. We know that the nominal stress is expressed as: P zz = ψ λ z where λ z = λ λ z. Therefore, applying
Homework 8 Model Solution Section
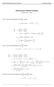 MATH 004 Homework Solution Homework 8 Model Solution Section 14.5 14.6. 14.5. Use the Chain Rule to find dz where z cosx + 4y), x 5t 4, y 1 t. dz dx + dy y sinx + 4y)0t + 4) sinx + 4y) 1t ) 0t + 4t ) sinx
MATH 004 Homework Solution Homework 8 Model Solution Section 14.5 14.6. 14.5. Use the Chain Rule to find dz where z cosx + 4y), x 5t 4, y 1 t. dz dx + dy y sinx + 4y)0t + 4) sinx + 4y) 1t ) 0t + 4t ) sinx
Areas and Lengths in Polar Coordinates
 Kiryl Tsishchanka Areas and Lengths in Polar Coordinates In this section we develop the formula for the area of a region whose boundary is given by a polar equation. We need to use the formula for the
Kiryl Tsishchanka Areas and Lengths in Polar Coordinates In this section we develop the formula for the area of a region whose boundary is given by a polar equation. We need to use the formula for the
Areas and Lengths in Polar Coordinates
 Kiryl Tsishchanka Areas and Lengths in Polar Coordinates In this section we develop the formula for the area of a region whose boundary is given by a polar equation. We need to use the formula for the
Kiryl Tsishchanka Areas and Lengths in Polar Coordinates In this section we develop the formula for the area of a region whose boundary is given by a polar equation. We need to use the formula for the
RECIPROCATING COMPRESSOR CALCULATION SHEET
 Gas properties, flowrate and conditions 1 Gas name Air RECIPRCATING CMPRESSR CALCULATIN SHEET WITH INTERCLER ( Sheet : 1. f 4.) Item or symbol Quantity Unit Item or symbol Quantity Unit 2 Suction pressure,
Gas properties, flowrate and conditions 1 Gas name Air RECIPRCATING CMPRESSR CALCULATIN SHEET WITH INTERCLER ( Sheet : 1. f 4.) Item or symbol Quantity Unit Item or symbol Quantity Unit 2 Suction pressure,
Phys460.nb Solution for the t-dependent Schrodinger s equation How did we find the solution? (not required)
 Phys460.nb 81 ψ n (t) is still the (same) eigenstate of H But for tdependent H. The answer is NO. 5.5.5. Solution for the tdependent Schrodinger s equation If we assume that at time t 0, the electron starts
Phys460.nb 81 ψ n (t) is still the (same) eigenstate of H But for tdependent H. The answer is NO. 5.5.5. Solution for the tdependent Schrodinger s equation If we assume that at time t 0, the electron starts
Second Order Partial Differential Equations
 Chapter 7 Second Order Partial Differential Equations 7.1 Introduction A second order linear PDE in two independent variables (x, y Ω can be written as A(x, y u x + B(x, y u xy + C(x, y u u u + D(x, y
Chapter 7 Second Order Partial Differential Equations 7.1 Introduction A second order linear PDE in two independent variables (x, y Ω can be written as A(x, y u x + B(x, y u xy + C(x, y u u u + D(x, y
wave energy Superposition of linear plane progressive waves Marine Hydrodynamics Lecture Oblique Plane Waves:
 3.0 Marine Hydrodynamics, Fall 004 Lecture 0 Copyriht c 004 MIT - Department of Ocean Enineerin, All rihts reserved. 3.0 - Marine Hydrodynamics Lecture 0 Free-surface waves: wave enery linear superposition,
3.0 Marine Hydrodynamics, Fall 004 Lecture 0 Copyriht c 004 MIT - Department of Ocean Enineerin, All rihts reserved. 3.0 - Marine Hydrodynamics Lecture 0 Free-surface waves: wave enery linear superposition,
Thi=Τ1. Thο=Τ2. Tci=Τ3. Tco=Τ4. Thm=Τ5. Tcm=Τ6
 1 Τ.Ε.Ι. ΑΘΗΝΑΣ / Σ.ΤΕ.Φ. ΤΜΗΜΑ ΕΝΕΡΓΕΙΑΚΗΣ ΤΕΧΝΟΛΟΓΙΑΣ ΕΡΓΑΣΤΗΡΙΟ ΜΕΤΑΔΟΣΗΣ ΘΕΡΜΟΤΗΤΟΣ Οδός Αγ.Σπυρίδωνος,12210 Αιγάλεω,Αθήνα Τηλ.: 2105385355, email: ptsiling@teiath.gr H ΕΠΙΔΡΑΣΗ ΠΑΡΟΧΗΣ ΟΓΚΟΥ ΣΤΗΝ
1 Τ.Ε.Ι. ΑΘΗΝΑΣ / Σ.ΤΕ.Φ. ΤΜΗΜΑ ΕΝΕΡΓΕΙΑΚΗΣ ΤΕΧΝΟΛΟΓΙΑΣ ΕΡΓΑΣΤΗΡΙΟ ΜΕΤΑΔΟΣΗΣ ΘΕΡΜΟΤΗΤΟΣ Οδός Αγ.Σπυρίδωνος,12210 Αιγάλεω,Αθήνα Τηλ.: 2105385355, email: ptsiling@teiath.gr H ΕΠΙΔΡΑΣΗ ΠΑΡΟΧΗΣ ΟΓΚΟΥ ΣΤΗΝ
Major Concepts. Multiphase Equilibrium Stability Applications to Phase Equilibrium. Two-Phase Coexistence
 Major Concepts Multiphase Equilibrium Stability Applications to Phase Equilibrium Phase Rule Clausius-Clapeyron Equation Special case of Gibbs-Duhem wo-phase Coexistence Criticality Metastability Spinodal
Major Concepts Multiphase Equilibrium Stability Applications to Phase Equilibrium Phase Rule Clausius-Clapeyron Equation Special case of Gibbs-Duhem wo-phase Coexistence Criticality Metastability Spinodal
Mock Exam 7. 1 Hong Kong Educational Publishing Company. Section A 1. Reference: HKDSE Math M Q2 (a) (1 + kx) n 1M + 1A = (1) =
 Mock Eam 7 Mock Eam 7 Section A. Reference: HKDSE Math M 0 Q (a) ( + k) n nn ( )( k) + nk ( ) + + nn ( ) k + nk + + + A nk... () nn ( ) k... () From (), k...() n Substituting () into (), nn ( ) n 76n 76n
Mock Eam 7 Mock Eam 7 Section A. Reference: HKDSE Math M 0 Q (a) ( + k) n nn ( )( k) + nk ( ) + + nn ( ) k + nk + + + A nk... () nn ( ) k... () From (), k...() n Substituting () into (), nn ( ) n 76n 76n
titanium laser beam volume to be heated joint titanium Fig. 5.1
 1 Lasers are often used to form precision-welded joints in titanium. To form one such joint it is first necessary to increase the temperature of the titanium to its melting point. Fig. 5.1 shows the joint
1 Lasers are often used to form precision-welded joints in titanium. To form one such joint it is first necessary to increase the temperature of the titanium to its melting point. Fig. 5.1 shows the joint
3.4 SUM AND DIFFERENCE FORMULAS. NOTE: cos(α+β) cos α + cos β cos(α-β) cos α -cos β
 3.4 SUM AND DIFFERENCE FORMULAS Page Theorem cos(αβ cos α cos β -sin α cos(α-β cos α cos β sin α NOTE: cos(αβ cos α cos β cos(α-β cos α -cos β Proof of cos(α-β cos α cos β sin α Let s use a unit circle
3.4 SUM AND DIFFERENCE FORMULAS Page Theorem cos(αβ cos α cos β -sin α cos(α-β cos α cos β sin α NOTE: cos(αβ cos α cos β cos(α-β cos α -cos β Proof of cos(α-β cos α cos β sin α Let s use a unit circle
Math 6 SL Probability Distributions Practice Test Mark Scheme
 Math 6 SL Probability Distributions Practice Test Mark Scheme. (a) Note: Award A for vertical line to right of mean, A for shading to right of their vertical line. AA N (b) evidence of recognizing symmetry
Math 6 SL Probability Distributions Practice Test Mark Scheme. (a) Note: Award A for vertical line to right of mean, A for shading to right of their vertical line. AA N (b) evidence of recognizing symmetry
( ) 2 and compare to M.
 Problems and Solutions for Section 4.2 4.9 through 4.33) 4.9 Calculate the square root of the matrix 3!0 M!0 8 Hint: Let M / 2 a!b ; calculate M / 2!b c ) 2 and compare to M. Solution: Given: 3!0 M!0 8
Problems and Solutions for Section 4.2 4.9 through 4.33) 4.9 Calculate the square root of the matrix 3!0 M!0 8 Hint: Let M / 2 a!b ; calculate M / 2!b c ) 2 and compare to M. Solution: Given: 3!0 M!0 8
the total number of electrons passing through the lamp.
 1. A 12 V 36 W lamp is lit to normal brightness using a 12 V car battery of negligible internal resistance. The lamp is switched on for one hour (3600 s). For the time of 1 hour, calculate (i) the energy
1. A 12 V 36 W lamp is lit to normal brightness using a 12 V car battery of negligible internal resistance. The lamp is switched on for one hour (3600 s). For the time of 1 hour, calculate (i) the energy
Problem Set 9 Solutions. θ + 1. θ 2 + cotθ ( ) sinθ e iφ is an eigenfunction of the ˆ L 2 operator. / θ 2. φ 2. sin 2 θ φ 2. ( ) = e iφ. = e iφ cosθ.
 Chemistry 362 Dr Jean M Standard Problem Set 9 Solutions The ˆ L 2 operator is defined as Verify that the angular wavefunction Y θ,φ) Also verify that the eigenvalue is given by 2! 2 & L ˆ 2! 2 2 θ 2 +
Chemistry 362 Dr Jean M Standard Problem Set 9 Solutions The ˆ L 2 operator is defined as Verify that the angular wavefunction Y θ,φ) Also verify that the eigenvalue is given by 2! 2 & L ˆ 2! 2 2 θ 2 +
DESIGN OF MACHINERY SOLUTION MANUAL h in h 4 0.
 DESIGN OF MACHINERY SOLUTION MANUAL -7-1! PROBLEM -7 Statement: Design a double-dwell cam to move a follower from to 25 6, dwell for 12, fall 25 and dwell for the remader The total cycle must take 4 sec
DESIGN OF MACHINERY SOLUTION MANUAL -7-1! PROBLEM -7 Statement: Design a double-dwell cam to move a follower from to 25 6, dwell for 12, fall 25 and dwell for the remader The total cycle must take 4 sec
2 Composition. Invertible Mappings
 Arkansas Tech University MATH 4033: Elementary Modern Algebra Dr. Marcel B. Finan Composition. Invertible Mappings In this section we discuss two procedures for creating new mappings from old ones, namely,
Arkansas Tech University MATH 4033: Elementary Modern Algebra Dr. Marcel B. Finan Composition. Invertible Mappings In this section we discuss two procedures for creating new mappings from old ones, namely,
Appendix to On the stability of a compressible axisymmetric rotating flow in a pipe. By Z. Rusak & J. H. Lee
 Appendi to On the stability of a compressible aisymmetric rotating flow in a pipe By Z. Rusak & J. H. Lee Journal of Fluid Mechanics, vol. 5 4, pp. 5 4 This material has not been copy-edited or typeset
Appendi to On the stability of a compressible aisymmetric rotating flow in a pipe By Z. Rusak & J. H. Lee Journal of Fluid Mechanics, vol. 5 4, pp. 5 4 This material has not been copy-edited or typeset
Higher Derivative Gravity Theories
 Higher Derivative Gravity Theories Black Holes in AdS space-times James Mashiyane Supervisor: Prof Kevin Goldstein University of the Witwatersrand Second Mandelstam, 20 January 2018 James Mashiyane WITS)
Higher Derivative Gravity Theories Black Holes in AdS space-times James Mashiyane Supervisor: Prof Kevin Goldstein University of the Witwatersrand Second Mandelstam, 20 January 2018 James Mashiyane WITS)
Contents of Appendix
 Contents of Appendix A SI UNITS: SINGLE-STATE PROPERTIES 755 Table A.1 Conversion Factors, 755 Table A.2 Critical Constants, 758 Table A.3 Properties of Selected Solids at 25 C, 759 Table A.4 Properties
Contents of Appendix A SI UNITS: SINGLE-STATE PROPERTIES 755 Table A.1 Conversion Factors, 755 Table A.2 Critical Constants, 758 Table A.3 Properties of Selected Solids at 25 C, 759 Table A.4 Properties
Strain gauge and rosettes
 Strain gauge and rosettes Introduction A strain gauge is a device which is used to measure strain (deformation) on an object subjected to forces. Strain can be measured using various types of devices classified
Strain gauge and rosettes Introduction A strain gauge is a device which is used to measure strain (deformation) on an object subjected to forces. Strain can be measured using various types of devices classified
UDZ Swirl diffuser. Product facts. Quick-selection. Swirl diffuser UDZ. Product code example:
 UDZ Swirl diffuser Swirl diffuser UDZ, which is intended for installation in a ventilation duct, can be used in premises with a large volume, for example factory premises, storage areas, superstores, halls,
UDZ Swirl diffuser Swirl diffuser UDZ, which is intended for installation in a ventilation duct, can be used in premises with a large volume, for example factory premises, storage areas, superstores, halls,
Homework 3 Solutions
 Homework 3 Solutions Igor Yanovsky (Math 151A TA) Problem 1: Compute the absolute error and relative error in approximations of p by p. (Use calculator!) a) p π, p 22/7; b) p π, p 3.141. Solution: For
Homework 3 Solutions Igor Yanovsky (Math 151A TA) Problem 1: Compute the absolute error and relative error in approximations of p by p. (Use calculator!) a) p π, p 22/7; b) p π, p 3.141. Solution: For
RECIPROCATING COMPRESSOR CALCULATION SHEET
 RECIPRCATING CMPRESSR CALCULATIN SHEET WITH INTERCLER Gas properties, flowrate and conditions 1 Gas name Air Item or symbol Quantity Unit Item or symbol Quantity Unit 2 Suction pressure, ps 1.013 bar A
RECIPRCATING CMPRESSR CALCULATIN SHEET WITH INTERCLER Gas properties, flowrate and conditions 1 Gas name Air Item or symbol Quantity Unit Item or symbol Quantity Unit 2 Suction pressure, ps 1.013 bar A
RECIPROCATING COMPRESSOR CALCULATION SHEET ISOTHERMAL COMPRESSION Gas properties, flowrate and conditions. Compressor Calculation Sheet
 RECIPRCATING CMPRESSR CALCULATIN SHEET ISTHERMAL CMPRESSIN Gas properties, flowrate and conditions 1 Gas name Air Item or symbol Quantity Unit Item or symbol Quantity Unit Formula 2 Suction pressure, ps
RECIPRCATING CMPRESSR CALCULATIN SHEET ISTHERMAL CMPRESSIN Gas properties, flowrate and conditions 1 Gas name Air Item or symbol Quantity Unit Item or symbol Quantity Unit Formula 2 Suction pressure, ps
4.4 Superposition of Linear Plane Progressive Waves
 .0 Marine Hydrodynamics, Fall 08 Lecture 6 Copyright c 08 MIT - Department of Mechanical Engineering, All rights reserved..0 - Marine Hydrodynamics Lecture 6 4.4 Superposition of Linear Plane Progressive
.0 Marine Hydrodynamics, Fall 08 Lecture 6 Copyright c 08 MIT - Department of Mechanical Engineering, All rights reserved..0 - Marine Hydrodynamics Lecture 6 4.4 Superposition of Linear Plane Progressive
TSOKOS CHAPTER 6 TEST REVIEW
 PHYSICS I HONORS/PRE-IB PHYSICS Name: DEVIL PHYSICS Period: Date: # Marks: XX Raw Score: IB Curve: BADDEST CLASS ON CAMPUS TSOKOS CHAPTER 6 TEST REVIEW S1. Thermal energy is transferred through the glass
PHYSICS I HONORS/PRE-IB PHYSICS Name: DEVIL PHYSICS Period: Date: # Marks: XX Raw Score: IB Curve: BADDEST CLASS ON CAMPUS TSOKOS CHAPTER 6 TEST REVIEW S1. Thermal energy is transferred through the glass
DETERMINATION OF THERMAL PERFORMANCE OF GLAZED LIQUID HEATING SOLAR COLLECTORS
 ΕΘΝΙΚΟ ΚΕΝΤΡΟ ΕΡΕΥΝΑΣ ΦΥΣΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΗΜΟΚΡΙΤΟΣ / DEMOKRITOS NATIONAL CENTER FOR SCIENTIFIC RESEARCH ΕΡΓΑΣΤΗΡΙΟ ΟΚΙΜΩΝ ΗΛΙΑΚΩΝ & ΑΛΛΩΝ ΕΝΕΡΓΕΙΑΚΩΝ ΣΥΣΤΗΜΑΤΩΝ LABORATORY OF TESTIN SOLAR & OTHER ENERY
ΕΘΝΙΚΟ ΚΕΝΤΡΟ ΕΡΕΥΝΑΣ ΦΥΣΙΚΩΝ ΕΠΙΣΤΗΜΩΝ ΗΜΟΚΡΙΤΟΣ / DEMOKRITOS NATIONAL CENTER FOR SCIENTIFIC RESEARCH ΕΡΓΑΣΤΗΡΙΟ ΟΚΙΜΩΝ ΗΛΙΑΚΩΝ & ΑΛΛΩΝ ΕΝΕΡΓΕΙΑΚΩΝ ΣΥΣΤΗΜΑΤΩΝ LABORATORY OF TESTIN SOLAR & OTHER ENERY
Partial Differential Equations in Biology The boundary element method. March 26, 2013
 The boundary element method March 26, 203 Introduction and notation The problem: u = f in D R d u = ϕ in Γ D u n = g on Γ N, where D = Γ D Γ N, Γ D Γ N = (possibly, Γ D = [Neumann problem] or Γ N = [Dirichlet
The boundary element method March 26, 203 Introduction and notation The problem: u = f in D R d u = ϕ in Γ D u n = g on Γ N, where D = Γ D Γ N, Γ D Γ N = (possibly, Γ D = [Neumann problem] or Γ N = [Dirichlet
b. Use the parametrization from (a) to compute the area of S a as S a ds. Be sure to substitute for ds!
 MTH U341 urface Integrals, tokes theorem, the divergence theorem To be turned in Wed., Dec. 1. 1. Let be the sphere of radius a, x 2 + y 2 + z 2 a 2. a. Use spherical coordinates (with ρ a) to parametrize.
MTH U341 urface Integrals, tokes theorem, the divergence theorem To be turned in Wed., Dec. 1. 1. Let be the sphere of radius a, x 2 + y 2 + z 2 a 2. a. Use spherical coordinates (with ρ a) to parametrize.
Lecture 2: Dirac notation and a review of linear algebra Read Sakurai chapter 1, Baym chatper 3
 Lecture 2: Dirac notation and a review of linear algebra Read Sakurai chapter 1, Baym chatper 3 1 State vector space and the dual space Space of wavefunctions The space of wavefunctions is the set of all
Lecture 2: Dirac notation and a review of linear algebra Read Sakurai chapter 1, Baym chatper 3 1 State vector space and the dual space Space of wavefunctions The space of wavefunctions is the set of all
[1] P Q. Fig. 3.1
![[1] P Q. Fig. 3.1 [1] P Q. Fig. 3.1](/thumbs/79/80362156.jpg) 1 (a) Define resistance....... [1] (b) The smallest conductor within a computer processing chip can be represented as a rectangular block that is one atom high, four atoms wide and twenty atoms long. One
1 (a) Define resistance....... [1] (b) The smallest conductor within a computer processing chip can be represented as a rectangular block that is one atom high, four atoms wide and twenty atoms long. One
Capacitors - Capacitance, Charge and Potential Difference
 Capacitors - Capacitance, Charge and Potential Difference Capacitors store electric charge. This ability to store electric charge is known as capacitance. A simple capacitor consists of 2 parallel metal
Capacitors - Capacitance, Charge and Potential Difference Capacitors store electric charge. This ability to store electric charge is known as capacitance. A simple capacitor consists of 2 parallel metal
Matrices and Determinants
 Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
Matrices and Determinants SUBJECTIVE PROBLEMS: Q 1. For what value of k do the following system of equations possess a non-trivial (i.e., not all zero) solution over the set of rationals Q? x + ky + 3z
Correction Table for an Alcoholometer Calibrated at 20 o C
 An alcoholometer is a device that measures the concentration of ethanol in a water-ethanol mixture (often in units of %abv percent alcohol by volume). The depth to which an alcoholometer sinks in a water-ethanol
An alcoholometer is a device that measures the concentration of ethanol in a water-ethanol mixture (often in units of %abv percent alcohol by volume). The depth to which an alcoholometer sinks in a water-ethanol
Approximation of distance between locations on earth given by latitude and longitude
 Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
Approximation of distance between locations on earth given by latitude and longitude Jan Behrens 2012-12-31 In this paper we shall provide a method to approximate distances between two points on earth
6.1. Dirac Equation. Hamiltonian. Dirac Eq.
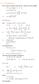 6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
6.1. Dirac Equation Ref: M.Kaku, Quantum Field Theory, Oxford Univ Press (1993) η μν = η μν = diag(1, -1, -1, -1) p 0 = p 0 p = p i = -p i p μ p μ = p 0 p 0 + p i p i = E c 2 - p 2 = (m c) 2 H = c p 2
Calculating the propagation delay of coaxial cable
 Your source for quality GNSS Networking Solutions and Design Services! Page 1 of 5 Calculating the propagation delay of coaxial cable The delay of a cable or velocity factor is determined by the dielectric
Your source for quality GNSS Networking Solutions and Design Services! Page 1 of 5 Calculating the propagation delay of coaxial cable The delay of a cable or velocity factor is determined by the dielectric
PETROSKILLS COPYRIGHT
 Contents Specific Gravity... 2 Formation Volume Factor... 3 Compressibility Equation... 4 Pseudo-Reduced Variables... 5 Pseudo-Critical Variables... 6 SI Conversions... 6 Output... 6 Input... 6 Viscosity...
Contents Specific Gravity... 2 Formation Volume Factor... 3 Compressibility Equation... 4 Pseudo-Reduced Variables... 5 Pseudo-Critical Variables... 6 SI Conversions... 6 Output... 6 Input... 6 Viscosity...
5.4 The Poisson Distribution.
 The worst thing you can do about a situation is nothing. Sr. O Shea Jackson 5.4 The Poisson Distribution. Description of the Poisson Distribution Discrete probability distribution. The random variable
The worst thing you can do about a situation is nothing. Sr. O Shea Jackson 5.4 The Poisson Distribution. Description of the Poisson Distribution Discrete probability distribution. The random variable
derivation of the Laplacian from rectangular to spherical coordinates
 derivation of the Laplacian from rectangular to spherical coordinates swapnizzle 03-03- :5:43 We begin by recognizing the familiar conversion from rectangular to spherical coordinates (note that φ is used
derivation of the Laplacian from rectangular to spherical coordinates swapnizzle 03-03- :5:43 We begin by recognizing the familiar conversion from rectangular to spherical coordinates (note that φ is used
FLAME-X 950 (N)HXCH FE180/E90 0,6/1kV DIN VDE 0266, DIN
 Halogen- free low smoke fire resistant security power cables with copper concentric conductor CONSTRUCTION Conductors: bare copper conductor, circular solid class 1 (RE) or stranded circular or circular
Halogen- free low smoke fire resistant security power cables with copper concentric conductor CONSTRUCTION Conductors: bare copper conductor, circular solid class 1 (RE) or stranded circular or circular
Section 8.3 Trigonometric Equations
 99 Section 8. Trigonometric Equations Objective 1: Solve Equations Involving One Trigonometric Function. In this section and the next, we will exple how to solving equations involving trigonometric functions.
99 Section 8. Trigonometric Equations Objective 1: Solve Equations Involving One Trigonometric Function. In this section and the next, we will exple how to solving equations involving trigonometric functions.
k A = [k, k]( )[a 1, a 2 ] = [ka 1,ka 2 ] 4For the division of two intervals of confidence in R +
[a 1, a 2 ] = [ka 1,ka 2 ] 4For the division of two intervals of confidence in R + k A = [k, k]( )[a 1, a 2 ] = [ka 1,ka 2 ] 4For the division of two intervals of confidence in R +](/thumbs/73/69566903.jpg) Chapter 3. Fuzzy Arithmetic 3- Fuzzy arithmetic: ~Addition(+) and subtraction (-): Let A = [a and B = [b, b in R If x [a and y [b, b than x+y [a +b +b Symbolically,we write A(+)B = [a (+)[b, b = [a +b
Chapter 3. Fuzzy Arithmetic 3- Fuzzy arithmetic: ~Addition(+) and subtraction (-): Let A = [a and B = [b, b in R If x [a and y [b, b than x+y [a +b +b Symbolically,we write A(+)B = [a (+)[b, b = [a +b
Μονοβάθμια Συστήματα: Εξίσωση Κίνησης, Διατύπωση του Προβλήματος και Μέθοδοι Επίλυσης. Απόστολος Σ. Παπαγεωργίου
 Μονοβάθμια Συστήματα: Εξίσωση Κίνησης, Διατύπωση του Προβλήματος και Μέθοδοι Επίλυσης VISCOUSLY DAMPED 1-DOF SYSTEM Μονοβάθμια Συστήματα με Ιξώδη Απόσβεση Equation of Motion (Εξίσωση Κίνησης): Complete
Μονοβάθμια Συστήματα: Εξίσωση Κίνησης, Διατύπωση του Προβλήματος και Μέθοδοι Επίλυσης VISCOUSLY DAMPED 1-DOF SYSTEM Μονοβάθμια Συστήματα με Ιξώδη Απόσβεση Equation of Motion (Εξίσωση Κίνησης): Complete
of the methanol-dimethylamine complex
 Electronic Supplementary Information for: Fundamental and overtone virational spectroscopy, enthalpy of hydrogen ond formation and equilirium constant determination of the methanol-dimethylamine complex
Electronic Supplementary Information for: Fundamental and overtone virational spectroscopy, enthalpy of hydrogen ond formation and equilirium constant determination of the methanol-dimethylamine complex
Solutions to Exercise Sheet 5
 Solutions to Eercise Sheet 5 jacques@ucsd.edu. Let X and Y be random variables with joint pdf f(, y) = 3y( + y) where and y. Determine each of the following probabilities. Solutions. a. P (X ). b. P (X
Solutions to Eercise Sheet 5 jacques@ucsd.edu. Let X and Y be random variables with joint pdf f(, y) = 3y( + y) where and y. Determine each of the following probabilities. Solutions. a. P (X ). b. P (X
Lifting Entry (continued)
 ifting Entry (continued) Basic planar dynamics of motion, again Yet another equilibrium glide Hypersonic phugoid motion Planar state equations MARYAN 1 01 avid. Akin - All rights reserved http://spacecraft.ssl.umd.edu
ifting Entry (continued) Basic planar dynamics of motion, again Yet another equilibrium glide Hypersonic phugoid motion Planar state equations MARYAN 1 01 avid. Akin - All rights reserved http://spacecraft.ssl.umd.edu
PETROSKILLS COPYRIGHT
 Contents Solution Gas-Oil Ratio... 2... 2... 2... 2 Formation Volume Factor... 3... 3... 3... 3 Viscosity... 4... 4... 4... 4 Density... 5 Bubble Point Pressure... 5... 6... 6... 6 Compressibility... 6
Contents Solution Gas-Oil Ratio... 2... 2... 2... 2 Formation Volume Factor... 3... 3... 3... 3 Viscosity... 4... 4... 4... 4 Density... 5 Bubble Point Pressure... 5... 6... 6... 6 Compressibility... 6
Other Test Constructions: Likelihood Ratio & Bayes Tests
 Other Test Constructions: Likelihood Ratio & Bayes Tests Side-Note: So far we have seen a few approaches for creating tests such as Neyman-Pearson Lemma ( most powerful tests of H 0 : θ = θ 0 vs H 1 :
Other Test Constructions: Likelihood Ratio & Bayes Tests Side-Note: So far we have seen a few approaches for creating tests such as Neyman-Pearson Lemma ( most powerful tests of H 0 : θ = θ 0 vs H 1 :
Group 30. Contents.
 Group 30 Contents Pump type Page Pump type Page Pump type Page 30A(C)...X002H 4 30A(C)...X013H 5 30A(C)...X068H 6 30A(C)...X068HU 7 30A(C)...X136H 8 30A(C)...X136Y 9 30A(C)...X146H 10 30A(C)...X160H 11
Group 30 Contents Pump type Page Pump type Page Pump type Page 30A(C)...X002H 4 30A(C)...X013H 5 30A(C)...X068H 6 30A(C)...X068HU 7 30A(C)...X136H 8 30A(C)...X136Y 9 30A(C)...X146H 10 30A(C)...X160H 11
ΚΥΠΡΙΑΚΗ ΕΤΑΙΡΕΙΑ ΠΛΗΡΟΦΟΡΙΚΗΣ CYPRUS COMPUTER SOCIETY ΠΑΓΚΥΠΡΙΟΣ ΜΑΘΗΤΙΚΟΣ ΔΙΑΓΩΝΙΣΜΟΣ ΠΛΗΡΟΦΟΡΙΚΗΣ 19/5/2007
 Οδηγίες: Να απαντηθούν όλες οι ερωτήσεις. Αν κάπου κάνετε κάποιες υποθέσεις να αναφερθούν στη σχετική ερώτηση. Όλα τα αρχεία που αναφέρονται στα προβλήματα βρίσκονται στον ίδιο φάκελο με το εκτελέσιμο
Οδηγίες: Να απαντηθούν όλες οι ερωτήσεις. Αν κάπου κάνετε κάποιες υποθέσεις να αναφερθούν στη σχετική ερώτηση. Όλα τα αρχεία που αναφέρονται στα προβλήματα βρίσκονται στον ίδιο φάκελο με το εκτελέσιμο
Econ 2110: Fall 2008 Suggested Solutions to Problem Set 8 questions or comments to Dan Fetter 1
 Eon : Fall 8 Suggested Solutions to Problem Set 8 Email questions or omments to Dan Fetter Problem. Let X be a salar with density f(x, θ) (θx + θ) [ x ] with θ. (a) Find the most powerful level α test
Eon : Fall 8 Suggested Solutions to Problem Set 8 Email questions or omments to Dan Fetter Problem. Let X be a salar with density f(x, θ) (θx + θ) [ x ] with θ. (a) Find the most powerful level α test
Free Energy and Phase equilibria
 Free Energy and Phase equilibria Thermodynamic integration 7.1 Chemical potentials 7.2 Overlapping distributions 7.2 Umbrella sampling 7.4 Application: Phase diagram of Carbon Why free energies? Reaction
Free Energy and Phase equilibria Thermodynamic integration 7.1 Chemical potentials 7.2 Overlapping distributions 7.2 Umbrella sampling 7.4 Application: Phase diagram of Carbon Why free energies? Reaction
ADVANCED STRUCTURAL MECHANICS
 VSB TECHNICAL UNIVERSITY OF OSTRAVA FACULTY OF CIVIL ENGINEERING ADVANCED STRUCTURAL MECHANICS Lecture 1 Jiří Brožovský Office: LP H 406/3 Phone: 597 321 321 E-mail: jiri.brozovsky@vsb.cz WWW: http://fast10.vsb.cz/brozovsky/
VSB TECHNICAL UNIVERSITY OF OSTRAVA FACULTY OF CIVIL ENGINEERING ADVANCED STRUCTURAL MECHANICS Lecture 1 Jiří Brožovský Office: LP H 406/3 Phone: 597 321 321 E-mail: jiri.brozovsky@vsb.cz WWW: http://fast10.vsb.cz/brozovsky/
ΠΣΤΥΙΑΚΗ ΔΡΓΑΙΑ. Μειέηε Υξόλνπ Απνζηείξσζεο Κνλζέξβαο κε Τπνινγηζηηθή Ρεπζηνδπλακηθή. Αζαλαζηάδνπ Βαξβάξα
 ΣΔΥΝΟΛΟΓΙΚΟ ΔΚΠΑΙΓΔΤΣΙΚΟ ΙΓΡΤΜΑ ΘΔΑΛΟΝΙΚΗ ΥΟΛΗ ΣΔΥΝΟΛΟΓΙΑ ΣΡΟΦΙΜΩΝ & ΓΙΑΣΡΟΦΗ ΣΜΗΜΑ ΣΔΥΝΟΛΟΓΙΑ ΣΡΟΦΙΜΩΝ ΠΣΤΥΙΑΚΗ ΔΡΓΑΙΑ Μειέηε Υξόλνπ Απνζηείξσζεο Κνλζέξβαο κε Τπνινγηζηηθή Ρεπζηνδπλακηθή Αζαλαζηάδνπ Βαξβάξα
ΣΔΥΝΟΛΟΓΙΚΟ ΔΚΠΑΙΓΔΤΣΙΚΟ ΙΓΡΤΜΑ ΘΔΑΛΟΝΙΚΗ ΥΟΛΗ ΣΔΥΝΟΛΟΓΙΑ ΣΡΟΦΙΜΩΝ & ΓΙΑΣΡΟΦΗ ΣΜΗΜΑ ΣΔΥΝΟΛΟΓΙΑ ΣΡΟΦΙΜΩΝ ΠΣΤΥΙΑΚΗ ΔΡΓΑΙΑ Μειέηε Υξόλνπ Απνζηείξσζεο Κνλζέξβαο κε Τπνινγηζηηθή Ρεπζηνδπλακηθή Αζαλαζηάδνπ Βαξβάξα
Second Order RLC Filters
 ECEN 60 Circuits/Electronics Spring 007-0-07 P. Mathys Second Order RLC Filters RLC Lowpass Filter A passive RLC lowpass filter (LPF) circuit is shown in the following schematic. R L C v O (t) Using phasor
ECEN 60 Circuits/Electronics Spring 007-0-07 P. Mathys Second Order RLC Filters RLC Lowpass Filter A passive RLC lowpass filter (LPF) circuit is shown in the following schematic. R L C v O (t) Using phasor
ΕΛΛΗΝΙΚΗ ΔΗΜΟΚΡΑΤΙΑ Ανώτατο Εκπαιδευτικό Ίδρυμα Πειραιά Τεχνολογικού Τομέα. Ξενόγλωσση Τεχνική Ορολογία
 ΕΛΛΗΝΙΚΗ ΔΗΜΟΚΡΑΤΙΑ Ανώτατο Εκπαιδευτικό Ίδρυμα Πειραιά Τεχνολογικού Τομέα Ξενόγλωσση Τεχνική Ορολογία Ενότητα: Principles of an Internal Combustion Engine Παναγιώτης Τσατσαρός Τμήμα Μηχανολόγων Μηχανικών
ΕΛΛΗΝΙΚΗ ΔΗΜΟΚΡΑΤΙΑ Ανώτατο Εκπαιδευτικό Ίδρυμα Πειραιά Τεχνολογικού Τομέα Ξενόγλωσση Τεχνική Ορολογία Ενότητα: Principles of an Internal Combustion Engine Παναγιώτης Τσατσαρός Τμήμα Μηχανολόγων Μηχανικών
Trigonometric Formula Sheet
 Trigonometric Formula Sheet Definition of the Trig Functions Right Triangle Definition Assume that: 0 < θ < or 0 < θ < 90 Unit Circle Definition Assume θ can be any angle. y x, y hypotenuse opposite θ
Trigonometric Formula Sheet Definition of the Trig Functions Right Triangle Definition Assume that: 0 < θ < or 0 < θ < 90 Unit Circle Definition Assume θ can be any angle. y x, y hypotenuse opposite θ
Example Sheet 3 Solutions
 Example Sheet 3 Solutions. i Regular Sturm-Liouville. ii Singular Sturm-Liouville mixed boundary conditions. iii Not Sturm-Liouville ODE is not in Sturm-Liouville form. iv Regular Sturm-Liouville note
Example Sheet 3 Solutions. i Regular Sturm-Liouville. ii Singular Sturm-Liouville mixed boundary conditions. iii Not Sturm-Liouville ODE is not in Sturm-Liouville form. iv Regular Sturm-Liouville note
Main source: "Discrete-time systems and computer control" by Α. ΣΚΟΔΡΑΣ ΨΗΦΙΑΚΟΣ ΕΛΕΓΧΟΣ ΔΙΑΛΕΞΗ 4 ΔΙΑΦΑΝΕΙΑ 1
 Main source: "Discrete-time systems and computer control" by Α. ΣΚΟΔΡΑΣ ΨΗΦΙΑΚΟΣ ΕΛΕΓΧΟΣ ΔΙΑΛΕΞΗ 4 ΔΙΑΦΑΝΕΙΑ 1 A Brief History of Sampling Research 1915 - Edmund Taylor Whittaker (1873-1956) devised a
Main source: "Discrete-time systems and computer control" by Α. ΣΚΟΔΡΑΣ ΨΗΦΙΑΚΟΣ ΕΛΕΓΧΟΣ ΔΙΑΛΕΞΗ 4 ΔΙΑΦΑΝΕΙΑ 1 A Brief History of Sampling Research 1915 - Edmund Taylor Whittaker (1873-1956) devised a
1 P age. Hydrogen-abstraction reactions of methyl ethers, H 3 COCH 3-x (CH 3 ) x, x=0 2, by OH; Chong-Wen Zhou C 3
 Table S1. Rotational constants and vibrational frequencies of Reactants, Complexes, Transition States and Products Computed at the MP2/6-311G(d,p) level. Species I a, I b, I c (GHZ) Frequencies (cm -1
Table S1. Rotational constants and vibrational frequencies of Reactants, Complexes, Transition States and Products Computed at the MP2/6-311G(d,p) level. Species I a, I b, I c (GHZ) Frequencies (cm -1
D Alembert s Solution to the Wave Equation
 D Alembert s Solution to the Wave Equation MATH 467 Partial Differential Equations J. Robert Buchanan Department of Mathematics Fall 2018 Objectives In this lesson we will learn: a change of variable technique
D Alembert s Solution to the Wave Equation MATH 467 Partial Differential Equations J. Robert Buchanan Department of Mathematics Fall 2018 Objectives In this lesson we will learn: a change of variable technique
EE512: Error Control Coding
 EE512: Error Control Coding Solution for Assignment on Finite Fields February 16, 2007 1. (a) Addition and Multiplication tables for GF (5) and GF (7) are shown in Tables 1 and 2. + 0 1 2 3 4 0 0 1 2 3
EE512: Error Control Coding Solution for Assignment on Finite Fields February 16, 2007 1. (a) Addition and Multiplication tables for GF (5) and GF (7) are shown in Tables 1 and 2. + 0 1 2 3 4 0 0 1 2 3
ANSWERSHEET (TOPIC = DIFFERENTIAL CALCULUS) COLLECTION #2. h 0 h h 0 h h 0 ( ) g k = g 0 + g 1 + g g 2009 =?
 Teko Classes IITJEE/AIEEE Maths by SUHAAG SIR, Bhopal, Ph (0755) 3 00 000 www.tekoclasses.com ANSWERSHEET (TOPIC DIFFERENTIAL CALCULUS) COLLECTION # Question Type A.Single Correct Type Q. (A) Sol least
Teko Classes IITJEE/AIEEE Maths by SUHAAG SIR, Bhopal, Ph (0755) 3 00 000 www.tekoclasses.com ANSWERSHEET (TOPIC DIFFERENTIAL CALCULUS) COLLECTION # Question Type A.Single Correct Type Q. (A) Sol least
DERIVATION OF MILES EQUATION FOR AN APPLIED FORCE Revision C
 DERIVATION OF MILES EQUATION FOR AN APPLIED FORCE Revision C By Tom Irvine Email: tomirvine@aol.com August 6, 8 Introduction The obective is to derive a Miles equation which gives the overall response
DERIVATION OF MILES EQUATION FOR AN APPLIED FORCE Revision C By Tom Irvine Email: tomirvine@aol.com August 6, 8 Introduction The obective is to derive a Miles equation which gives the overall response
The Simply Typed Lambda Calculus
 Type Inference Instead of writing type annotations, can we use an algorithm to infer what the type annotations should be? That depends on the type system. For simple type systems the answer is yes, and
Type Inference Instead of writing type annotations, can we use an algorithm to infer what the type annotations should be? That depends on the type system. For simple type systems the answer is yes, and
Supplementary Materials for. Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides
 Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
Supplementary Materials for Kinetic and Computational Studies on Pd(I) Dimer- Mediated Halogen Exchange of Aryl Iodides Indrek Kalvet, a Karl J. Bonney, a and Franziska Schoenebeck a * a Institute of Organic
ω ω ω ω ω ω+2 ω ω+2 + ω ω ω ω+2 + ω ω+1 ω ω+2 2 ω ω ω ω ω ω ω ω+1 ω ω2 ω ω2 + ω ω ω2 + ω ω ω ω2 + ω ω+1 ω ω2 + ω ω+1 + ω ω ω ω2 + ω
 0 1 2 3 4 5 6 ω ω + 1 ω + 2 ω + 3 ω + 4 ω2 ω2 + 1 ω2 + 2 ω2 + 3 ω3 ω3 + 1 ω3 + 2 ω4 ω4 + 1 ω5 ω 2 ω 2 + 1 ω 2 + 2 ω 2 + ω ω 2 + ω + 1 ω 2 + ω2 ω 2 2 ω 2 2 + 1 ω 2 2 + ω ω 2 3 ω 3 ω 3 + 1 ω 3 + ω ω 3 +
0 1 2 3 4 5 6 ω ω + 1 ω + 2 ω + 3 ω + 4 ω2 ω2 + 1 ω2 + 2 ω2 + 3 ω3 ω3 + 1 ω3 + 2 ω4 ω4 + 1 ω5 ω 2 ω 2 + 1 ω 2 + 2 ω 2 + ω ω 2 + ω + 1 ω 2 + ω2 ω 2 2 ω 2 2 + 1 ω 2 2 + ω ω 2 3 ω 3 ω 3 + 1 ω 3 + ω ω 3 +
An experimental and theoretical study of the gas phase kinetics of atomic chlorine reactions with CH 3 NH 2, (CH 3 ) 2 NH, and (CH 3 ) 3 N
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 An experimental and theoretical study of the gas phase kinetics of atomic chlorine
Proses = 0 / 0 Proses = 0 / 36 16" 4576 / 2.3 Barat : 4833 / Utara : 5941 / 3.05 Proses = 63 / 37 Flow : 9936 / 3.2
 A BALANCE Proses = 120 / 31 391 t/h T Gun = 29 T Gun = 31 [38oC] A-101-JCA 443 / 2.46 10" TG = 31 05 November 2012 Rate 102.075% Proses = 0 / 33 349 t/h T Gun = 29 T Gun = 37 [38oC] A-101-JCB 261 / 1.42
A BALANCE Proses = 120 / 31 391 t/h T Gun = 29 T Gun = 31 [38oC] A-101-JCA 443 / 2.46 10" TG = 31 05 November 2012 Rate 102.075% Proses = 0 / 33 349 t/h T Gun = 29 T Gun = 37 [38oC] A-101-JCB 261 / 1.42
ΚΥΠΡΙΑΚΟΣ ΣΥΝΔΕΣΜΟΣ ΠΛΗΡΟΦΟΡΙΚΗΣ CYPRUS COMPUTER SOCIETY 21 ος ΠΑΓΚΥΠΡΙΟΣ ΜΑΘΗΤΙΚΟΣ ΔΙΑΓΩΝΙΣΜΟΣ ΠΛΗΡΟΦΟΡΙΚΗΣ Δεύτερος Γύρος - 30 Μαρτίου 2011
 Διάρκεια Διαγωνισμού: 3 ώρες Απαντήστε όλες τις ερωτήσεις Μέγιστο Βάρος (20 Μονάδες) Δίνεται ένα σύνολο από N σφαιρίδια τα οποία δεν έχουν όλα το ίδιο βάρος μεταξύ τους και ένα κουτί που αντέχει μέχρι
Διάρκεια Διαγωνισμού: 3 ώρες Απαντήστε όλες τις ερωτήσεις Μέγιστο Βάρος (20 Μονάδες) Δίνεται ένα σύνολο από N σφαιρίδια τα οποία δεν έχουν όλα το ίδιο βάρος μεταξύ τους και ένα κουτί που αντέχει μέχρι
ΚΥΠΡΙΑΚΗ ΕΤΑΙΡΕΙΑ ΠΛΗΡΟΦΟΡΙΚΗΣ CYPRUS COMPUTER SOCIETY ΠΑΓΚΥΠΡΙΟΣ ΜΑΘΗΤΙΚΟΣ ΔΙΑΓΩΝΙΣΜΟΣ ΠΛΗΡΟΦΟΡΙΚΗΣ 24/3/2007
 Οδηγίες: Να απαντηθούν όλες οι ερωτήσεις. Όλοι οι αριθμοί που αναφέρονται σε όλα τα ερωτήματα μικρότεροι του 10000 εκτός αν ορίζεται διαφορετικά στη διατύπωση του προβλήματος. Αν κάπου κάνετε κάποιες υποθέσεις
Οδηγίες: Να απαντηθούν όλες οι ερωτήσεις. Όλοι οι αριθμοί που αναφέρονται σε όλα τα ερωτήματα μικρότεροι του 10000 εκτός αν ορίζεται διαφορετικά στη διατύπωση του προβλήματος. Αν κάπου κάνετε κάποιες υποθέσεις
Parametrized Surfaces
 Parametrized Surfaces Recall from our unit on vector-valued functions at the beginning of the semester that an R 3 -valued function c(t) in one parameter is a mapping of the form c : I R 3 where I is some
Parametrized Surfaces Recall from our unit on vector-valued functions at the beginning of the semester that an R 3 -valued function c(t) in one parameter is a mapping of the form c : I R 3 where I is some
Srednicki Chapter 55
 Srednicki Chapter 55 QFT Problems & Solutions A. George August 3, 03 Srednicki 55.. Use equations 55.3-55.0 and A i, A j ] = Π i, Π j ] = 0 (at equal times) to verify equations 55.-55.3. This is our third
Srednicki Chapter 55 QFT Problems & Solutions A. George August 3, 03 Srednicki 55.. Use equations 55.3-55.0 and A i, A j ] = Π i, Π j ] = 0 (at equal times) to verify equations 55.-55.3. This is our third
