KIRISh Termodinamika fani nazariy fizikaning asosiy bo`limlaridan biri xisoblanadi. Termodinamika fani muvozanat xolatda bo`lgan termodinamika
|
|
|
- Μυρίνη Βυζάντιος
- 7 χρόνια πριν
- Προβολές:
Transcript
1 KIRIh ermodnamka fan nazary fzkanng asosy bo`lmlardan br xsoblanad. ermodnamka fan muvozanat xolatda bo`lgan termodnamka sstemalarnng ssklk blan boxlangan umumy xususyatlarn, konunyatlarn va unda utayotgan jarayonlarn tekshrad va o`rganad. Bu erda kkta muxm narsadan foydalanlad:. tatstk metod asosda olngan umumy konunyatlardan, formulalardan, mkdory fodalardan va xulosalardan.. ajrba asosda olngan muxm natjalardan. hunng uchun xam termodnamka asosan ssklknng xarakat konunlarn o`rganuvch fandr. ekshrsh masalasga karab, termodnamka 3 ksmga bo`lnad:. Fzkavy termodnamka.. Kmyovy termodnamka. 3. exnkavy termodnamka. Fzkavy termodnamka termodnamkanng umumy nazary asoslar va aksomalarn o`rganad. Kmyovy termodnamka esa kmyovy va fzkavy muvozanatlarn tekshrshda termodnamkanng nazary asoslardan va metodlardan foydalanad. exnkavy termodnamka esa ssklk va shn o`zaro br-brga almashnshn o`rganshda termodnamkanng asosy konunlardan foydalanad. exnkavy termodnamkan asosy maksad ssklk mashnalarnng nazaryasn shlab chkshdan borat. ermodnamkada asosan o`n btta tushuncha mavjud:. ermodnamk sstema yok makroskopk sstema.. ermodnamk sstema xolat. 3. ermodnamk muvozanat. 4. ermodnamk jarayon. 5. emperatura. 6. Energyan saklansh va aylansh konun. 7. ermodnamk sstema chk energyas. 8. ermodnamk sh. 9. Issklk mkdor. 0. Xolatfunktsyas.. Xolattenglamas. ermodnamkada kkta tekshrsh metod kullanlad:. Doravy termodnamk jarayonlar metod.. ermodnamk potentsallar metod. Doravy jarayonlar metod frantsuz a`d Karno va nems Klauzus tomondan shlab chklgan. ermodnamk potentsal metod esa amerkalk Gbbsga tegshldr. ermodnamkanng asosda uchta konun yotad:. ermodnamkanng brnch konun chk energya tuxrsdag konun.. ermodnamkanng kknch konun entropya tuxrsdag konun. 3. ermodnamkanng uchnch konun yok Nernstnng ssklk teoremas.
2 I. MUOZANADAGI IEMALAR ERMODINAMIKAI.. ERMODINAMIKADA AOIY UHUNHALAR. Makroskopk sstema Katta sondag zarralardan tashkl topgan xar kanday jsmga makroskopk sstema deylad. Makroskopk sstema o`lcham xar dom atom va molekula o`lchamdan katta bo`lad.. ermodnamk sstema xolat Makroskopk sstema xolatn makroskopk parametrlar (bosm, xajm, zchlk, elastklk, kutblansh, magntlansh va x.k.z.) anklayd. Makroskopk parametrlar tashk va chk parametrlarga ajralad. Ichk parametrlar o`z navbatda ntensv va ekstensv parametrlarga ajratlad. Agar parametrlar sstema massas va undag zarralar songa boxlk bo`lmasa ntensv parametrlar deb, agar massa va zarralar songa proportsonal bo`lsa ekstensv yok addtv parametrlar deb yurtlad. 3. ermodnamk muvozanat Agar sstema parametrlar vakt o`tsh blan o`zgarmasa, bunday xolat statsonar deylad. Bundan tashkar, sstema parametrlar vakt bo`ycha o`zgarmas bo`lbgna kolmay, kandaydr tashk manbalar ta`sr xsobda xech kanday statsonar okmlar bo`lmasa, u xolda bunday sstema muvozanat xolatda deylad (yok termodnamk muvozanat xolatda deylad). Muvozanat xolatda sstemada katta vakt oralx yuzaga kelad. Fzka materyanng struktur ko`rnshlarga mos keluvch xarakatnng eng oddy shakldag konunyatlarn o`rganad. Ularn br xolatdan kknch xolatga aylanshda, bu xarakat shakllarnng umumy o`lchovga energya deb yurtlad. ermodnamk sstemalar zolyatsyalangan va zolyatsyalanmagan bo`lad. ashk jsm blan o`zaro ta`srlashmaydgan (energya, modda, nurlansh blan) sstemaga zolyatsyalangan sstema deylad. Izolyatsyalangan sstemada termodnamk muvozanat xolat mavjud bo`lad. Bu muvozanat xolat vakt o`tsh blan yuzaga kelad va xech vakt o`z xolcha ana shu muvozanat xolatdan chka olmayd. Bunga termodnamkanng brnch yok asosy postulat deb yurtlad. Bu termodnamkanng brnch dastlabk fkr termodnamkanng umumy boshlansh deb xam yurtlad.
3 4. ermodnamk jarayon ermodnamk sstemanng br muvozanat xolatdan kknch muvozanat xolatga utshga termodnamk jarayon deb yurtlad. ermodnamkada kkta jarayon fark klnad:. Kvazstatstk (jarayon).. Nokvazstatstk (kaytmas) jarayon. 5. emperatura ajrba shun ko`rsatadk, termodnamk muvozanat ssklk xarakatnng maxsus kurnsh sfatda xam yuzaga kelar ekan. Agar kkta muvozanatdag sstemalar ssklk kontaktga keltrlsa, u xolda tashk parametr λ n farkga yok tenglgga karamasdan, ular lgargdek termodnamk muvozanat xolat kolad yok muvozanat xolat ularda buzlad va ma`lum vakt o`tgandan so`ng ssklk almashnsh (energya almashnsh tufayl) jarayonda kkala sstema boshka muvozanat xolatga o`tad. Bundan tashkar, agar uchta muvozanat xolatdag sstemalar bo`lsa va brnch va kknch sstemalarnng xar br uchnch sstema blan muvozanat xolatda bo`lsa, u xolda brnch va kknch sstemalar xam o`zaro termodnamk muvozanat xolatda bo`lad (termodnamk muvozanatnng tranztvlk xususyat). Demak, sstemanng termodnamk muvozanat xolat fakat tashk parametrlar λ blan anklanmasdan, ssteman chk xolatn xarakterlovch, yana btta kattalk t blan anklanad turl xl muvozanatdag sstemalarn ssklk kontaktda, davomylgda va olganda xam energya almashnsh natjasda t nng kymat br xl bo`lb kolad. Bu fkr shunday xulosaga olb keladk, agar boshka bror jsmn foydalansak, termodnamk muvozanat xolatnng tranztvlk xossas turl xl sstemalarn to`xrdan to`xr o`zaro ssklk kontaktga keltrmasdan turb, t nng kymatn solshtrsh mkonyatn berad. Odatda, ana shu kattalk t ga temperatura deylad, muvozanatdag sstema xolatnng maxsus funktsyas sfatda temperaturan mavjudlg to`xrsdag fkrga termodnamkanng kknch dastlabk fkr deb yurtlad. Odatda, bunga nolnch boshlansh xam deb yurtlad. emperaturan mavjudlg ta`srdag fkrn kuydagcha ta`rflash mumkn. Bz yukorda ko`rdkk, termodnamk sstemanng muvozanat xolat tashk va chk parametrlar blan xarakterlanad, shu blan brga chk parametrlar sstema molekulalarnng urnga va xarakatga va tashk parametrlarnng kymatga boxlk bo`lad. emperaturan mavjudlg to`xrsdag fkr esa termodnamk muvozanat xolat tashk parametrlar to`plam va temperatura blan anklanshn tklayd. Demak, chk parametrlar sstema xolatn xarakterlamasdan muvozanatdag sstemanng boxlanmagan parametrlar bo`la olmayd. hunday klb, sstemanng xamma muvozanatl chk parametrlar tashk parametrlar va temperaturanng funktsyasdr (termodnamkanng kknch postulat). stema energyas unng chk parametrdr, u vaktda muvozanatda energya tashk parametr va temperatura funktsyasdr. Bu funktsyadan temperaturan
4 energya va tashk parametr orkal fodalab, termodnamkanng kknch dastlabk fkrn kuydagcha ta`rflash mumkn. ermodnamk muvozanatdan sstemanng chk parametrlar tashk parametrlar va energyanng funktsyasdr. ermodnamkanng kknch dastlabk fkr jsm temperatura o`zgarshn, unng kays br chk parametrn o`zgarsh bo`ycha anklash mkonn berad, (shunga asoslanb) turl xl termometrlarnng kurlsh shunga asoslangan. raktk xolatda temperaturan anklashda modda blan boxlangan kandaydr ank shkala blan foydalanshga to`xr kelad. ermometrk parametr sfatda odatda, shu modda xajm foydalanlad, shkala uchun esa sel`sya bo`ycha tanlanad. emperatura Kel`vn shkalas bo`ycha xaì o`lchanad. + t 73, 5 + t α (..) t - temperatura sel`sy shkalas bo`ycha olngan α ³aæm kengaysh koefftsent. 6. Energyan saklansh va aylansh konun aðakatnng yo`kolmaslg va unng br xarakat formasdan boshka br xarakat formasga o`tshga ya`n saklansh va aylansh konun deylad. Energyanng saklansh va aylansh konun: Gess (840 yl), Joul (840 yl), Mayer (84 yl) va Gel`mgol`ts (847 yl) tomondan tklangan. Energyan saklash va aylansh konun mkdory va sfat ko`rnshlarga ega. ermodnamkada energyan saklansh va aylansh konun ssklk jarayonlar uchun tadbk klshda olngan mkdory fodas termodnamka brnch konunnng mkdory fodasn berad. Umuman xarakat shakllarnng umumy o`lchovga energya deylad. 7. ermodnamk sstemnnga chk energyas að kanday termodnamk sstema katta sondag zarralardan tashkl topgan. Uzluksz xarakatlanuvch va o`zaro ta`srlanuvch ana shu zarralar energyasga termodnamk sstema energyas deylad. stema to`la energyas tashk va chk energyaga ajralad. steman br butun xolda xarakat energyas va tashk kuch maydondag potentsal energyasga tashk energya deylad. stemanng kolgan bo`lak energyalarga chk energya deylad. Masalan, N ta zarradan tashkl topgan real gaz chk energyas e kuydag ko`rnshga ega bo`lad: N E + U + m j N ( r r ) U ( r ). (..)
5 Bu erda - - zarra mpul`s, U ( r r ) energyas, va j - zarralarnng o`zaro ta`sr ( zarran tutgan urn blan boxlangan potentsal energya. U r ) Ichk energya e sstema chk parametr bo`lb xsoblanad. Demak, chk energya sstema muvozanat xolatda tashk parametrlar λ va temperatura ga boxlk bo`lad: E ( λ λ, λ,... λ ) E (..3), 3 n 8,9. ermodnamk sh. Issklk mkdor ermodnamkada sh tushunchas muxm rol` o`ynayd, chunk sstema xolat o`zgargandagna termodnamk sh bajarlad. µaraladgan sstema tashk jsm blan o`zaro ta`srda bo`lgandagna, sstema xolat o`zgarad va natjada shn mkdory tomondan anklash mumkn bo`lad. Xakkatan xam, sstema noldan farkl sh bajarsh uchun, u albatta tashk jsmlarn sljtsh kerak. ajrba shun ko`rsatadk, termodnamk ssteman, un o`rab olgan muxt blan o`zaro ta`srda energya almashnsh yuz berad. Bu erda energyan sstemadan tashk jsmlarga uzatsh kkta xar xl usulda bo`lsh mumkn. stema tashk parametrlarnng o`zgarsh blan boxlk bulsh va bu parametrlarnng o`zgarshsz boxlk bulsh. ashk parametrlarnng o`zgarsh blan boxlk bo`lgan energya uzatshn brnch usulga sh deylad. ashk parametrlarnng o`zgarshsz, ammo yang termodnamk parametr (entropya)nng o`zgarsh blan boxlk bo`lgan energya uzatshn kknch usulga ssklk, energya uzatsh jarayonnng bu usulga ssklk almashnsh deylad. ashk parametrlarnng o`zgarsh blan sstemaga berlgan energyaga sh deylb, A xarf blan belglanad. ashk parametrlarnng uzgarshsz sstemaga berlgan energyaga ssklk mkdor deylb, δ ³arf blan belglanad. Bajarlgan sh δ A t dλ (..4) formula yordamda toplad. Bu erda δa - cheksz kchk bajarlgan sh, t - umumlashgan kuch, λ - umumlashgan parametr. ermodnamkada bajarlgan shn shoras kuydagcha kabul klngan. Agar sstema tashk kuchlarga karsh sh bajarsa musbat, agar tashk sstema ustda tashk kuchlar sh bajarsa manfy yok sstema kengaysh jarayonda bajarlgan shn fodalovch yuza jarayon yo`nalshn fodalovch egrlkdan o`ng tomonda yotsa musbat agar chap tomonda yotsa manfy deb xam kabul klngan. Bu fkrlardan shu narsa kelb chkadk, sstema br xolatdan kknch xolatga o`tganda bajarlgan kengaysh yok sklsh sh o`tsh yo`lga karab, o`zgarb turar ekan. Ya`n, bajarlgan shn kattalg o`tsh yo`lga boxlk bo`lad. Bu esa bajarlgan sh jarayon funktsyas bo`lshn ko`rsatad. hunng uchun bajarlgan sh
6 δa ko`rnshda ya`n to`lkmas dfferentsal ko`rnshda yozlad. Masalan, agar sstema kengaysh sh bajarayotgan bo`lsa δa- pdv, agar sklsh sh bajarlayotgan bo`lsa δa- pdv ko`rnshda yozlad. Agar tashk elektr maydon ta`sr ostda zotrop delektrk ustda kutblash sh bajarlayotgan bo`lsa: δa εd bo`lad. Bu erda ε - tashk elektr maydon kuchlanganlg, - kutblansh vektor. Agar tashk magnt maydon magntk ustda magntlash kuch bajarlayotgan bo`lsa: δa HdM bo`lad. Bu erda H - magnt maydon kuchlanganlg, M - magntlansh vektor. 0. Xolatfunktsyas uzaro boxlanmagan mkroskopk parametrlar to`plam sstema xolatn anklayd. Berlgan vaktda sstema ³îlatn to`la xolda anklovch va sstemalarga xolat funktsyas deylad.. îëatnng termk va kalork tenglamalar ermodnamkanng kknch dastlabk fkrdan muvozanatl chk parametrlar va temperaturanng funktsyas bo`lshdan sstema xolatnng termk va kalork tenglamalarnng mavjudlgga olb kelad, ya`n temperaturada va tashk parametrlar λ kandaydr muvozanatl chk parametr b k blan boxlovch tenglama olb kelad. ( λ,..., λ ) bk f n, (..5) Agar chk parametr va chk energya E(b k E) bo`lsa, u xolda tenglama E( λ,..., λ ) (..6) E n, Energya tenglamas yok xolatnng kalork tenglamas deylad. hunday nomlanshga sabab, bu tenglama yordamda kaloryada fodalanuvch ssklk sxm va boshka shunga o`xshagan kattalklarn topsh mumkn. Agar chk parametr va tashk parametrga λ- ko`shma bo`lgan umumlashgan kuch f bo`lsa, u xolda tenglama f f (λ,, λn; ) (,,, n) ³îlatnng termk tenglamas deb yurtlad. Bunday nom blan yurtlshga sabab bu tenglamalar yordamda temperaturan xsoblash mumkn. îëatnng termk va kalork tenglamalarnng umumy son unng ozodlk darajalarnng songa teng bo`lad, ya`n sstema xolatn xarakterlovch boxlanmagan parametrlar songa teng bo`lad. Agar xolatnng termk va kalork tenglamalar ma`lum bo`lsa, u xolda termodnamkanng boshlanshlar yordamda sstemanng xamma termodnamk xususyatlarn anklash mumkn. ermodnamkanng boshlanshlarga asoslanb
7 xolat tenglamalarn chkarsh mumkn emas. Ular yok tajrbadan tklanad yok statstk fzka metod yordamda toplad. Bu xol esa termodnamka va statstk fzkas br brn to`ldrshn va ularn tamoman ajratsh mumkn emaslgn ko`rsatad. Muvozanatl sstemalarnng xususyatn o`rganshda, termodnamka eng avval, oddy sstema deganda kkta parametr blan anklanuvch br fazal sstemalarga aytlad. Oddy sstema xolatnng termk va kalork tenglamalar mos ravshda kuydag ko`rnshn olad: f E f ( λ ) (..7) E( λ ) (..8) Agar f p - bosm, λ - - sstema xajm bo`lsa, u xolda sstema xolatnng tenglamalar p p (, ) (..9) EE (, ) bo`lad. Ideal gaz uchun xolatnng termk tenglamas Klapeyron-Mendeleev tenglamas bo`lad. R R ν m µ R - br mol` gaz uchun (..0) mol` gaz uchun: m - gaz massas, µ - molyar massa. uzãarmas temperaturada deal gaz chk energyasn unng xajmga boxlk emaslsh to`xrsda Djoul konundan foydalansak, ya`n 0 U xolda deal gaz kalork tenglamasn olamz. E (..) E E E de, d + d d, E cd + E0 E (..) Br atoml deal gaz uchun e v + e 0 bo`lad. Ideal real gazlar uchun emprk xolda xolatnng 50 dan oshk termk tenglama tklangan: an-der-aals tenglamas
8 a + ( b) R (..3) b molekulyarnng xususy xajm. π b (..4) N, 0 Z0 3 a - chk bosm (.3) tenglamaga real gazlar uchun tuzatma krtshn brnch marta M..Lomonosov aytad (ssklknng tabat to`xrsda molekulyar knetk tasavvurga asoslanb). a ( b) Re R (.5) Dterchenng I tenglamas (..5) а + R 5/ 3 ( b) Dterchenng II tenglamas (..6) а + R ( b) Bertlo tenglamas. (..7) B D R (.6) ³îëatnng veral (..8) formadag tenglamas. Bu erda,,d. temperatura funktsyas bo`lb, ularga veral koefftsentlar deb yurtlad. Brnch xad deal gazga mos kelad, kaysk molekulalar orasda o`zaro ta`sr yo`kdr. Ikknch esa molekulalar orasdag juft to`knashshn xsobga olad va x.k.z. Real gazlarga molekulalar orasdag o`zaro ta`sr kuch kska ta`sr xarakterdalgn xsobga olb, Mayer va Bogolyubov turl xl metodlar yordamda xolat tenglamasn kuydagcha olad: p + R n Bn, n (..9) Bu erda vral koefftsentlar n gaz zarralar orasda o`zaro ta`sr potentsal orkal fodalanad. Masalan, agar molekulalar orasdag potentsal U fakat molekulalar orasdag masofa r nng funktsyas bo`lsa, u xolda N ta zarradan tashkl topgan gaznng kknch veral koefftsent
9 4() B( ) π N e K r dr. (..0) 0 () n ekspermentda o`lchab, o`zaro ta`srnng potentsal funktsyasnng parametrlarn topsh mumkn. stema xolat tenglamalarnng mavjudlgdan uchta termk koefftsentlar (kengaysh, sklsh, bosm elastklk) orasda kuydag boxlansh borlgn olsh mumkn: α 0 βγ. (..) 0 va R da sstema xajm va bosm. 0, β 0, γ α (..).. IIQLIK IG`IMI Reja. ermodnamk xarorat.. Issklk sxm. 3. Energya prntsp. ermodnamkanng I konun. 4. ermodnamkanng I prntsp tadbk. 5. Gazlarnng ssklk sxm. Moddanng ssklk sxm deb () jsmga berlgan elementar ssklk mkdornng δ Q shunga mos keluvch xarorat o`zgarshga nsbatga (d) aytlad: С δq (..) d Issklk sxm jsmnng massas va kmyovy tarkbga boxlk. Bundan tashkar, bu mkdor termodnamk xolatga ssklk bersh jarayonga xam boxlk. xrtacha ssklk sxm > bo`lganda jsm xaroratn gacha oshrshga sarflangan ssklk mkdorga aytlad: Q (..) Issklk sxm blan o`rtacha ssklk sxm orasdag boxlansh: 0
10 d (..3) Massa brlgdag ssklk sxm solshtrma ssklk sxm deylad. Br jnsl modda uchun с M N ta gaz aralashmas uchun, M jsm massas. N m c, c, m lar solshtrma ssklk sxm va komponent massa komponent. Atom ssklk sxm µ deb oddy modda klogramm atom (gramm- atom) ssklk sxmga aytlad: µ µc, µ - modda molyar oxrlg. mol` gaz chk energyas 3 И N Ak (..4) m massal gaz uchun И 3 µ N Ak (..5) m Molyar ssklk sxm ( const) Сµ И 3 3 кал kn A R 3 моль град (..6) Modda xaroratn dan + d gacha orttrshda sarflangan elementar ssklk mkdor kuydagda teng bo`lad. Br jnsl modda uchun δ Q di (..7) M δ Q Mcd µ d (..8) µ Oddy kmyovy modda uchun: M Q A p d δ (..9).3. ENeRGIYa RINsII. ermodinamikaning BIRINhI KONUNI Makroskopk xarakatsz sstemalar uchun bu konun ssklk jarayonlarda energyanng saklansh konunn fodalayd:
11 δ Q du + δa (.3.) δa - tashk kuchlar bajargan sh, δq - sstemaga berlgan ssklk mkdor, du chk energyaga o`zgarsh. Agar δq, du va δa lar turl kattalklarda o`lchanadgan bo`lsa, u xolda chk energya o`zgarsh (.3.) d U δ Q + δa j j j ssklk mkdor mexank ekvvalent: j 4,8 j/kal 0, 47 kgm/kal 0,39 kal/j,34 kal/kgm sh brlgdag ssklk ekvvalent deylad. j stemada elementar o`zgarsh sodr bo`lsa: δ Q du + δa (.3.3) yok d du + δa (.3.4) sstema ssklk sxm. Bunda tashk kuchlarga karsh bajarlgan sh u xolda I prntsp: δ A d + da* (.3.5) yok d du + d + δa* (.3.6) d dh dp + da* (.3.7) N- sstema entalpyas. Br komponentl br fazal modda uchun bajarlgan sh: U xolda: (, ) ва H f ( ) δ A * 0, U f, d U U d + + d (.3.8) d U H d + p d (.3.9) Izoxork jarayonda ssklk sxm ( const)
12 const U (.3.0) p H U + U F + U (.3.) Ideal gaz uchun va UM/(), U 0 va M R µ ρ M, µ lar -gaz massas va molyar massas. e - ssklk effektn brnch prntsp asosda tasvrlasak: U U pd H H + dp E (.3.) e - U U -U, e r - NN N ya`n o`zgarmas xajmda chk energya, o`zgarmas bosmda ental`pya yo`lnng shaklga emas balk boshlanxch va oxrg nuktalar vazyatga boxlk. Yukordaglarn xsobga olb I prntspn yozsak: du δ Q δa δq d (.3.3) δq d entropya formulasdan δ Q d n xsobga olb chk energyan du d d (.3.4) Brnch prntspn energya prntsp deb atalad. Ichk energyan tashk kuchlar bajargan sh δa va ssteman bron ssklk manbas blan tutashtrsh orkal o`zgartrsh mumkn. Keyng formulalar kaytarml va kaytarshsz muvozanatl sstemalar uchun xam o`rnldr. Oxrg formula xarorat, entropya va energya prntsplarnng o`zaro boxlanshn xarakterlayd. Bu tenglama asosy termodnamk tenglk deb yurtlad. Izotermk potentsal FU- dan U-F foda F- srt erkn energyas blan boxlangan energya orasdag boxlanshn bldrad. Izotermk jarayonda chk energyanng mkdorga kamayshn bldrad. stemaga berlgan ssklk mkdor rasmda ko`rsatlgandek, koordnat sstemasda tasvrlansa, jadvalda xosl bo`lgan yuzaga teng bo`lad. () Bun analtk usulda fodalasak:
13 Q d (.3.5) Bu erda δq d mkdor rasmdag yo`lnng boshlanxch va oxrg nuktalar vazyatga boxlk bo`lmay balk, kontur bo`ycha ntegralga boxlk bo`lad. hu sababl, bu ntegral ostdag foda to`lk dfferentsal emas. δq chk energyanng kandaydr mkdorga ortshn yok kamayshn bldrad va dq esa, - elementar ssklk mkdordan fark klad. url zo-jarayonlarda bajarlgan sh sngar sstemaga berlgan yok undan olngan ssklk mkdorn anklaymz: Izotermk jarayon: ( ) Entropya va Boyl`-Marot konunlardan foydalanb: hosl bo`lad: Q d (.3.6) R γ 0 l n, 0 (.3.7) 0 γ γ 0 0 Q γ R ln Rln γ γ 0 0 (.3.8) Izobark jarayon: const γ d d va ( γ ) Q p γ d γ γ γ d ( ) ( ) (.3.9) γ γ γ γ va Izoxork jarayon: (4.6) formuladan Q Adabatk jarayon: Q 0 Rd const td ( γ ) ( ) R d R ( ) γ γ γ (.3.0)
14 .4. GAZNING IIQLIK IG IMI Gaznng xaroratn 0 oshrsh uchun sarflanadgan ssklk mkdor ssklk sxm deylad: δq (.4.) δ Issklk sxm xam ssklk mkdor sngar jarayon funktsyasdr. Bu funktsya gaznng ssh shartga boxlk: adabatk jarayonda: δq0, 0 Izoxork jarayon: δqd ga asosan, (.4.), n xsoblaymz. topsak: R + θ (4.5) dan bo`ycha dfferentsallab entropyan 0 R γ l n ( θ ) ( θ ) γ R ln γ ( θ ) ( θ ) γ γ γ γ γ Br marta const deb, kknch marta const deb (8.) topamz: R, γ θ θ 0 bo`lsa, va lar o`zgarmas bo`lad. Bundan R Rγ, γ γ Rγ ( γ ) ( θ ) (.4.3) (.4.4) γ (.4.5) R (.4.6) (8.6) Mayer tenglamas deb yurtlad..5. BIRINHI RININING IZOROELARGA ADBIKI. Izoxork jarayon: const A0 Bu I prntsp ko`rnsh. ( ) δ Q du d; Q (.5.)
15 . Izobark jarayon: const δ Q du + Ish teng bo`lad: d Rd d d + d pd δ Q ( + R) d d d + Rd + R (.5.) const const Izobark jarayonda ssklk mkdor: R A ( ) W A ( ) Q ; Q H H (.5.3) ssklk mkdor ental`pya o`zgarshga teng. 3. const Izotermk jarayonda I prntsp kuydagcha anklanad: δ Q d + d (.5.4) d 0 ; δq d dw (.5.5) Bunda Q W
16 4. Adabatk jarayon: δ Q 0 d Q W R Rln (.5.6) d + d 0 ; Rd d + d (.5.7) d d, R, γ p p ; n ntegrallab d d γ (.5.8) γ ln ln (.5.9) dan γ γ const (.5.0) uasson tenglamas kelb chkd. µattk va suyuk jsmlar uchun: Gaz atomlar uchun: 5 R 0, 785 Ж моль R 8,33 j kal; (.5.) D`yulang t anklashcha, 6,4 kal/g.atm.gr.6,7 j/g.atom.gr. 5. I prntspnng elektromagnt maydonga, elektr xodsalarga tadbk. Gel`mgol`ts kuchlarnng saklansh noml asarda I prntspnng elektr va elektromagnt xodsalarga tadbkn bayon etgan. Elektr va magnt xodsalar uchun tadbk: tok kuch I, t - vaktda o`tkazgch orkal o`tsa, dt - vaktda o`tkazgch syd chk energyas uzgarad:du εidt (9.) ε manba e.yu.k. Ajralgan ssklk: dq I Rdt dw (.5.) Ichk energya Ikknch tomondan du dq + dw (.5.3) ε Idt I Rdt + dw (.5.4)
17 dw magnt maydon energyas. xtkazgch magnt maydonga krtlganda o`zaro ta`sr energyas: dw IdΦ, df magnt okm. Energya saklansh konunga asosan: ε Idt I Rdt + IdΦ (.5.5) Mos ravshda e.yu.k., tok kuch va nduktson e.yu.k teng: dφ ε dφ dt dφ ε IR + ; I ; ε (.5.6) dt R dt.6. ermodinamika II RINsII. KLAUZIU A`RIFI. ENROIYa xzgarihining IeMA IIKLIGIGA BO LIµLIGI tatstk fzkada entropya uchun (.6.) KlnW Funktsya xzmat klad. K, Ж ; W -termodnamk extmollk. 0 entropya. Yopk sstema entropyas К ( E,λ) Kl nω (.6.) energya va tashk parametrlarga boxlk kvant xolatlar son Ω (E,λ) energya extmollg blan kvant xolatlar son orasdag boxlansh: W ( ε ) Ω Ω ε ( ε ) e ( ε ) ε / θ ε / e θ ε / e θ dω( ε ) Z (.6.3) Bun kanonk taksmot deb atalad. stema entropyas kanonk taksmotnng o`rtacha kymatga teng: Xolatextmollg o`rtacha kymat Entropya o`zgarsh esa: ( E,λ) k l n Ω (.6.4) E U bo`lsa, u xolda entropya: ( U,λ) k l m Ω (.6.5)
18 d k l nω du + lnω dλ (.6.6) U λ λ σ θ E (0.7) ekanlgn xsobga olb belglasak. l nω( E, λ) (0.8) Bunda λ - tashk parametr, θ - statstk xarorat. (0.6) E θ dag fodan end sodda ko`rnshda yozsak: Bunda (0.6 ) dan k k d du + λdλ θ θ (.6.6 ) λ θ lnω (.6.9) λ θ du d λdλ (.6.0) k λ - parametr orkal vujudga kelgan natjavy kuch λ : ( du δq δa) θ d k δq yok kδq d (.6.) θ θ k (.6.); u xolda d δq (.6.3) (0.3) formula fenomenologk termodnamkada entropya tenglamas bo`lb shu orkal entropyaga ta`rf xam berlad, ya`n sstemaga berlgan ssklk mkdor sstema entropyasn o`zgartshga sarflanad. Yopk kontur bo`ycha entropya o`zgarsh ntegral Φ d 0 (.6.4) dq Φ 0. (0.5)
19 hu sababl entropya o`ssh to`lk dffrentsal ekan. Muvozanatsz jarayonlarda entropya ortsh konun Mkroxolat termodnamk extmollg W ; mkroxolat kvant xolatlar son Ω, orasdag boxlansh Muvozanatsz ssteman juda kchk sstemalarga bo`lamz W Ω (.6.6) τ << t << τ 0 stemaga relaksatsya vakt τ, ssteman kuzatsh vakt t, umumy sstema relaksatsya vakt τ 0. ermodnamk extmollk W Muvozanatl sstema entropyas Kvant xolatlar son (.6.7) ( W ) e Kln( W ) e ; (.6.8) kl nω (.6.9) kchk sstema entropyas. Kl nw KlnΩ (.6.0) Ω Ω (.6.) Yopk sstemada: M M 3; M - muvozanatl va M-3 -muvozanatsz sstema entropyasdan ortk bo`lad..7. AYLANMA JARAYoNLAR..KARNO sikli. koordnat sstemasda kaytarml muvozanatl jarayonn (-rasm, a) ko`rb o`taylk. Aytaylk grafkda tasvrlangandek, gaz xolat soat strelkas yo`nalshda aylanma tsklda xarakatlansn.
20 A d (.7.) a d b c b a c a) 3-расм. d б) Bajarlgan shga asosan tsklnng abc ksmda gaz kengaysh sodr bo`lad va musbat sh bajarlad, unng kymat abc egr chzk o`rab olgan yuzaga teng bo`lad. sda yo`nalshda teskar yo`nalshda gaz sklad tashk kuchlar ta`srda manfy sh bajarlad va unng kymat abc chzx xosl klgan yuza absolyut kymatga teng bo`lad. abc yo`nalshdag bajarlgan sh blan cda yo`nalshdag shlar fark abcda yopk kontur yuzga fark klad. 3-rasm b)da koordnat δq sstemasda a b c d a kontur yuzas orkal tasvrlangan. a b c ksmda d ga asosan entropya ortad gazga ssklk berlad - unng kymat a b c egrlk yuz orkal anklanad s d a ksmda gaz entropyas kamayad, ssklkn gazdan olnad va unng absolyut kymat c d a egr srt yuzasga teng. Bu kkala karamakarsh yo`nalshdag shlar fark a b c d a kontur yuz orkal anklanad energya prntspga ko`ra chk energya gaz xolat funktsyas φdu 0, u xolda φδ Q φdu + φδa (.7.) Q A (.7.3). hunday klb, R, tekslklarda tskllar yuz br-brga teng bo`lad. hunday klb, 3-rasm, a, b, da tasvrlangan nuktanng soat strelkas yo`nalshdag xarakatdag xar kanday ssklk mashnasnng ssklk uzatshdag bajarlgan shn xarakterlayd.
21 иситгич г а з совутгич иситгич Q AQ - Q Q AQ - Q г а з Q Q совутгич Issklk mashnas foydal sh koeftsent (FIK) А Q Q η (.7.4) Q Q Karno tskl R, tekslklarda tasvrlanshn ko`rb o`taylk. Kamtarml Karno tskl uchun FIK Q ( - ) ga asosan Q ( - ), Q ( - ) Bundan Q Q ; Q > 0, Q Q < 0 η (.7.5) a) 4-расм. б). (.7.3) ga asosan Karno tskl FIK shch modda turga boxlk emas.. Karno tskl FIK stgch va sovutgch xaroratlar nsbatga boxlk. \ kchk bo`lsa, Karno FIK shuncha katta bo`lad. 3. Karno FIK η < bo`lad. da bo`lganda η bo`lad a ; kknch tartbl abady dvgatel ko`rsh mumkn emas.
22 a) 5-расм. 3 5-rasmda tasvrlangan a, b tskllar uchun gaz shch modda sfatda karalb:.tskl barcha ksmlar uchun ssklk mkdor va bajarlgan shn topng..q A ekanlgn sbotlang. 3. tskl FIK n topng. Javob: skl, a). QA ( - )- p (.7.6) / γ 3. / γ ( ) ( ) η γ (.7.7) tskl, b). QAR( - ) l n ( 3 / ) (.7.8) 3. R( ) ln( 3 ) ( ) + R ln( ) η (.7.9) p 3
23 INO AOLLARI. Energya prntsp asosda I, II termodnamk prntsplarn tushuntrng.. Qaytar va qaytmas jarayonlar, muvozanatl va nomuvozanat sstema. 3. emperatura prntsp moxyat. 4. ermodnamk sh. 5. Ichk energya fzk moxyat. 6. Ichk energya blan ental`pya boxlansh. 7. Bog lansh energyas xarakterstkas. 8. F U- nng moxyat. 9. Izojarayonlarda bajarlgan sh. 0. Izojarayonlarda ssklk mkdor.. Entropya prntsp. II prntsp..8. ermodinamika AKIOMAIKAI. IXIYoRIY ermodinamik IeMADA ENROIYa UhUNhAINI UMUMLAhIRIh. NeRN RINsII. ermodinamika III RINsII.ermodnamk aksomalar asosan klassk termodnamkan bayon etshda keng ko`llanlad. hu aksomalar asosda termodnamk prntsplar, konunlar, mantky bayon etlad. Bunng uchun kuydagcha postulatlardan fundamental xoyalarn bayon etshda foydalanlad: 0.ermodnamkanng noll prntsp-temperatura mavjudlg xakdag postulatdr. Bu postulat logk jxatdan termodnamkada muxm xsoblansada, ko`pchllk ktoblarda undan foydalanlmagan. 0.ermodnamkanng brnch prntsp energya saklansh konunnng ssklk jarayonlarga tadbkdr: du δq - δa (.8..) 3 0.ermodnamka kknch prntsp sovuk jsmdan ssk jsmga ssklkn o`tkazb bo`lmaslgga kaytmas termodnamk jarayonlarn ekvvalent tarzda turlcha ta`rflarda bayon etlshn bayon etad. Un tarxy turlcha formulrovkalarda zoxlanad: Karno tskl, Kel`vn ta`rfda, Ostval`d formulas orkal, Klauzus postulatlar orkal: a) sstema ustda bron o`zgarsh klmay turb, past xaroratl jsmdan yukor xaroratl jsmga ssklkn o`tkazb bo`lmayd; b) xech kanday o`zgarshsz jsm ssklgn shga aylantrb bo`lmayd (okean suv ssklg); v) kknch tartbl abady dvgatel yasab bo`lmayd. Bu ekvvalent ta`rflarn muvozanatl sstemalar uchun xolat funktsyalar entropya uchun ssklk mkdor blan boxlk munosabatn entropya orkal:
24 δ Q d (.8..) I va II prntspn umumlashtrb yozsak: du d d (.8.3) Ifodanng o`ng tomon to`lk dfferentsallk sharotga ko`ra (, ) (, ) (, ) (, ) (.8.4) (, ) (, ) 0 Absolyut xarorat va absolyut entropya kalbrovkas kuydagcha bo`lgan termodnamk sstema mavjud: (, ) (, ) (, ) (, ) (, ) (, ) ga asosan dan (, ) (, ) (.8.5) (.8.5) foda kalbrovka shartn bldrad. Umumlashgan prntsp: du d d (.8.6) uíã tomon to`lk dfferentsaldr adabatk jarayon uchun dδa adabatk potentsal U chk energyaga teng bo`lad: du δ Q δa δq d (.3.) δ Q d (.3.) (, ) (, ) (.8.7) I Faraz klaylk brlashgan sstemadan δq ssklk mkdor kchk sstemalarga δq, δq mkdorda xar brga berlsn. δ Q δq + δq (.8.8)
25 Adabatk jarayonda δq 0, dσ 0 kchk mkdorda ssklk berlganda δq, δq, δq, proportsonal bo`lad shartn entropyaga; dσ, dσ, dσ bu koefftsentlar xolat parametrlarga boxlk bo`lb, musbat kymatga ega. δq f ( τ σ, x ) dσ, δq f ( τ, σ, x ), dσ δq f ( τ, σ, x ) dσ (.8.9) (.8.8) n (.8.9) ga ko`yb: f( τ, σ, x ) ( τ, σ, x, x ) d σ + f ( τ, σ, x ) ( τ, σ, x, x ) dσ dσ (.8.0) f f x parametr τ va σ boxlk emaslgdan: 0.σ fakat σ, σ nng funktsyas σ σ (σ, σ ) 0.f, f, f koefftsentlar temperatura va entropya shartga boxlk, x, x ga boxlk emas ( τ σ ), f f ( τ, σ ), f ( τ, σ ) f f, f (.8.) 3 0. f (τ, σ )/f(τ, σ) va f (τ, σ )/f(τ, σ) nsbat τ ga boxlk emas. Oxrg xossadan kelb chkad. Bundan topamz f f f f f τ τ 0, τ f f τ τ ( nf ) ( lnf ) Ω( τ ) l (bunda l nf σ ga boxlk emas, lnf esa σga ). U xolda (, ) (.8.) f ( τ, σ) Ψ( τ ) F ( σ), f( τ, σ ) Ψ( τ ) F ( σ ) f ( τ, σ ) Ψ( τ ) F ( σ ) (.8.3) Bunda Ψ( ) [ ( τ ) dx] τ exp Ω bu τ ³aðoratnng xoslavy funktsyas. (.8.3) n (.8.0) ga ko`yb topamz ( σ ) dσ F ( σ) dσ F ( σ ) dσ F + (.8.4)
26 End absolyut entropyan F( ) σ dσ va absolyut temperaturan Ψ(τ) formula orkal topamz. Bunng uchun (.8.9) va (.8.3) formuladan topamz δq/d. Bu II prntspnng odatdag formulas (.8.4) esa, absolyut entropyan bldrad. d d + (.8.5) + d, (.8.3) formula orkal absolyut xarorat va absolyut entropyanng kanchalk anklk darajasn ko`rb o`taylk. Aytaylk, kktadan absolyut temperatura va entropya mavjud bo`lsn. U xolda (.8.3) ga asosan: δ Q d yok d ( τ ) ( τ ) ( σ ) ( σ ) d (.8.6) d (.8.6) nng chap tomon fakatgna τ ga, o`ng tomon σ ga boxlk bo`lb, xar kkala tomon xam domy songa teng. (a) va ( τ ) a ( τ ); d( σ ) d( σ ); ( σ ) ( σ ) + b (.8.7) a σ Absolyut temperatura absolyut entropya anklk darajasn oshrshda xar kkalasnng xam bo`lnsh darajasn br xl a va a - deb br xl belglashga to`xr kelad. III. Ixtyory termodnamk sstemalar uchun entropya tushunchasn I va II prntspnng umumlashgan xolatdan foydalanb umumlashtrshmz mumkn. d δq/ formuladan foydalanb xar kanday sstema entropya shkalasn darajalash mumkn. II RINsI - NeRN RINsII Bu prntsp kmyovy termodnamkaga tegshl bo`lganlg sababl termodnamka ramkasda ayrm tajrbalar natjalarn umumlashtrlngan xolda postulatlar tarzda bayon etsh va un sbotlash mkonyatga ega emas. Nernst prntspn statstk fzka nukta nazardan kvanto-mexank tasavvurlar asosda sbotlash mumkn. xozr esa, fakatgna Nernst prntsp ta`rf va unng termodnamk okbatlar blan cheklanamz. Nernst prntsp III termodnamk prntsp: absolyut xarorat 0 bo`lganda xar kanday termodnamk sstemanng entropyas o`zgarmas bo`lb bronta o`zgaruvchan parametrlar: bosm, xajm, maydon kuchlanganlg sngarlarga boxlk emas. Ko`p xollarda bu domy kymat nolga teng. hu sababl Nernst
27 prntspn ko`p xollarda / 0 0 deb ta`rflanad. Lekn bu formula unversal xarakterga ega emas. Nernst prntspdan kator muxm xulosalar kelb chkad: 0. að kanday ssklk sxm 0 da nolga teng. xakkatan xam xarorat nolga ntlganda entropya kuydagcha fodalansh mumkn: 0 + A x (I) ( ) ( ) ( ) * (0) o`zgarmas mkdor xech kanday o`zgaruvchan parametrga boxlk emas, x parametr ssklk sxmn xsoblashda ko`llanladgan o`zgarmas (,R, va boshkalar). ( ) na( x) * С х formulaga asosan bundan x (0) bo`lganda xajm kengaysh koefftsent 0 (, ) xam asosy termodnamk tenglk: dan xosl klamz:, ( ) bo`lad. xakkatan (, ) (, ) (, ) (, ) 0 0 (II) hunk, 0 bo`lganda entropya bosmga boxlk bo`lmayd bo`lsa bosmnng termk koefftsent nolga teng bo`lad: (, ) (, ) (, ) (, ) 0 0 (III) 4.Absolyut nolga ershb bo`lmayd. Nernst teoremas ko`p xollarda kuydag sababga ko`ra absolyut nolga ershb bo`lmaslg xakdag prntsp xam atashad. Karno tskln ko`z oldmzga keltrsak, sovutgch 0 ³aðoratga ega bo`lsa, bunday kaytarml mashnanng to`lk entropya o`zgarsh tsklda zotermk o`zgarshga teng bo`lar ed : δq Q (I) Izotermk sovushda br ksmda 0 da zoentropya sodr bo`lad 0; kolgan kk jarayon zotermk xsoblanad. sklda to`la zoentropya o`zgarsh φ d 0 ()
28 Bunng karshlkka uchrash (Q 0) noll zotermanng shonchszlgn bldrad. 0 kaysk br vaktnng o`zda zoentropya (adabata) xam xsoblanad. 0, yok const. Ma`lumk, Karno tskl 0 temperatura va oxrg yuza blan umuman -tekslgda tasvrlansh mumkn emas. xakkatan xam zoterma zoentropya 0, 0 - tekslg boshlanxch nuktasda buzlad aynyd. Karno tskln tasvrlovch to`xr burchak xam temperatura o`kda buzlad. Isbotlangan bu xakkat 0 stalgancha yakn ntlshn ta`kklamayd..9. ENROIYa A UNING XOALARI KARAeODORI RINsII Entropya xam chk energya sngar xolat parametrlar funktsyas xsoblanad, sstemanng dastlabk va oxrg xolatlarga boxlk bo`lad, ammo yo`lnng shaklga boxlk emas. Ayrm xollarda entropyan termodnamk xolatlar mustakl parametrlar (R,,, ) sfatda va xolat parametrlar sngar karalad. δq µaytarml jarayonda: 0 - entropya olngan yok berlgan ssklk mkdorga ± δq ga boxlk bo`lad: Agar δq ssklk kabul klsa,+ entropya ortad, agar δq ssklk bersa-chkarsa, - entropya kamayad. δq 0 bo`lsa, const zoentropya deb atalad. Entropya o`lcham () noank o`zgarmas o`lcham blan mos tushad. Bu o`lchashnng boshlanxch shartlar ank bo`lmagan noank o`zgarmas kattalk. µaytarml jarayonlar uchun I II termodnamk prntsplarn brlashtrb yozsak: yok Q d du + d δ (.9.) d U + A da (.9.) mustakl o`zgaruvchan parametrlar U,; δqd u xolda (.9.) dan ga xosl klamz: d du + d (.9.3) Bunda entropya o`zgarsh d - to`lk dfferentsal. ³îëat funktsyas ntegral ko`paytmada absolyut xaroratda shtrok etad. mol` deal gaz entropyasnng a (R, ); v (R, ) nuktalardag o`zgarshn ko`rb o`taylk. d:
29 a) termodnamk jarayon a v yo`nalshda kchk xolatlarda yakn nuktalarda xsoblash shlarn bajaramz (3-rasm); a) jarayon a s s v yo`nalshda zotermk va zoxork xolat o`zgarshlarda sodr bo`lsn (6-rasm). xolat entropyas a s zoxork jarayon uchun Q d d ac ac δ (.9.4) entropya s v zotermada: d Q d Rd d δ cb R (.9.5) cb entropyanng a v tsklda umumy o`zgarsh d d Rd d d ac + cb (.9.6) ab + Entropyanng umumy kymat d ab - o`tsh yo`l formasga boxlk emas. hu sababl bu kymat to`lk dfferentsal deb atalad. yok d d + R + const n + R n + const l l (.9.7) l n + Rln + const (.9.7 ) const - noank o`zgarmas mkdor. (.9.7 ) tenglama Klapeyron tenglamas deb yurtlad. Un xolat parametrlarn xsobga olgan xolda kk marta ko`llab entropya o`zgarshn topamz: a lna + Rlna + const b lnb + Rlna + const b + b b a ln Rln a a (.9.8) Klapeyron tenglamasn ko`llab (.9.7) dan xaæmn chkarb, entropyan (R, ) xolat funktsyas sfatda karasak: Bunda p l n Rln + const (.9.9)
30 + R (.9.0). Entropya dagrammalar (, ) sarflangan ssklk mkdorn xsoblashda ko`llanlshn ko`rb o`taylk. ermodnamk jarayon 7-rasmda ko`rsatlgandek av a v yo`nalshda sodr bo`lsn. Unga (ava v ) mkdorda ssklk sarflansn a v ksmga: b Q d (.9.) ab a Bundan tashkar () dagrammada br termodnamk tskl bajarlganda sarflangan ssklk mkdor (7 rasm) Q d (.9.) AB A δq b A B a D a d b 7-rasm. A hakl yuzasga teng; (AD A ) blan A Q d (.9.3) DA (AD) yuza fark br tsklda bajarlgan teng bo`lad. url xolatdag gazlarn (,) koordnatalarda fodalasak: a) zotermk jarayon const (,) koordnata sstemasda to`xr chzkn fodalayd. b) adabata tenglamas const ordnata o`kga parallel bo`lad. (4 rasm)
31 Izobara va zoxora tenglamalarn (, ) koordnatalar uchun yozamz: l n + Rln + const (.9.4) d + const dan K, e / (.9.5) zoxork jarayonda xarorat eksponentsal konun asosda o`zgarad. const изотерма const адиабата 8 rasm v) const. Izobark jarayonda Klapeyron tenglamasn deal gaz uchun (,) koordnatalarda yozamz: ln + R 0 ln + const (.9.6) ( + R) n + const n const l l (.9.7) + e / (.9.7) dan K 0 (.9.8) > ; Adabatk jarayonn analz ko`rsatadk. II prntspga yangcha ta`rf bersh mumkn. Bun Karateodor (909 y.) tomondan tavsya etlad. Adabatk xolatn xosl klb bo`lmayd degan prntspn tavsya etad. d δq U U d + + d 0 (.9.8) Adabata uchun δq 0 (.9.8) tenglama faffa tenglamasga aylanad:
32 U d + U + d 0 (.9.9) d 0, entropya xolat funktsyas xsoblanad. (,) const (,) const (.9.0). (.9.0) foda adabata tenglamas deb atalad.,, 3 zoentropk xolat berlgan. a, v, s undag mos nuktalar. ³îëatdan ³îëatga adabatk o`zgarsh yo`l blan o`tb bo`lmayd. Ya`n adabatk jarayon o`zgarsh orkal a nuktadan v nuktaga, undan s nuktaga o`tb bo`lmayd. b c a 3 9 rasm.0. NeRN eoremai (KAN xolalari AOIDA) Ma`lumk, kvant xolatlar uchun Gbbsnng katta kanonk taksmot muvozanatl ansambl uchun kvant xolatlar extmollg. F E ω n n exp (.0.) K Ω E n, N + µ N ωn, N exp (.0.) K 0 eng kchk energetk kvant xolatn bldrad. tatstk oxrlk br brlkka ntlad. 0 (amalda bun olb bo`lmayd) stema entropyas unng statstk oxrlg logarfm nolga teng bo`lad. Bu xulosa kvant statstkasdan kelb chkad. (dskretlk) va Nernst teoremas yok termodnamka III prntspda o`z fodasn topgan. hu asosda 0 turl termodnamk kattalklar xaraktern anklash mkonn berad.
33 d Q δ (.0.3) d Q δ (.0.4) (.0.3), (.0.4) formuladan: 0 bo`lganda 0 (.0.5) Bundan tashkar 0 0, 0. U xolda dan 0 bo`lad. 0 bo`lsa. hunga o`xshash tarzda ( ) 0, pd d df (.0.6) munosabatdan (.0.7) end 0 da 0 (.0.8) odatda entropya 0, 0 ³aðorat nolga ntlganda darajal konunyat blan anklanad: n a (.0.9) Bosm yok gaz funktsyas (a), kandaydr parametr (n); 0 bo`lganda, termodnamk kattalklar ma`lum darajada noldan farkl bo`lad. hunday klb: n+ ; yok ( ) 0, ~ + С n (.0.0) Nernst teoremas ntegral domys kymatn topsh mkonn berad. ( ) ( ) d dq (.0.) n
34 const. bo`ycha ntegrallab ega bo`lamz: d Bunda xech kanday (.0.) ntegral domys yo`k, shtrok 0 W klmayd. n bosm o`zgarmas bo`lganda bo`ycha ntegrallab topamz: W W0 + d (.0.) 0 Bunda W 0 -entropyanng 0 bo`lgandag kymat. Bu fodan (W) va entropyan () (.0.) GW- formulaga ko`yb ega bo`lamz: G d W0 + d (.0.3) 0 0 G Gbbs termodnamk potentsal yok Gbbs energyas taby o`zgaruvchlar va R nng xarakterstk funktsyasdr.bunda ntegral domys 0 dan kutuldk, lekn yang W 0 - parametr entropyanng 0 dag kymat unng o`rnn old...ermodinamik KAALIKLARNING ZARRALAR ONIGA BO LIµLIGI Modda mkdorga boxlk termodnamk kattalklar: F, Ω, W, G br jnsl sstemada ekstensv modda mkdorga proportsonal xarakterga ega. Ekstensv kattalk ekstensv kattalkka nsbatan brnch darajal br jnsl funktsya bo`lsh kerak. f ekstensv funktsya, u, x.. x n - ekstensv o`zgaruvchan parametrlar. U xîëda tenglk bajarlsh kerak. a- xtyory son. (..) n х n (,..., n) ( y x x n ) xn ( y ax,;... ax ) af ( x ) f,... (..), n x n bo`ycha dfferentsallab ega bo`lamz: ( y ax,..., axn ) ( axn ) f ( y, x xn ) xn f,...,, (..) f,,... noll tartbl br jnsl funktsya.(..) n a bo`ycha dfferentsallab va (..) n xsobga olb ega bo`lamz:
35 n [ f ( y x,..., x ) x ] x f ( y, x,... x ), n n n n (..3) End (..) va (..3) fodalar kanday termodnamk funktsyalarn fodalashn ko`rb o`taylk. Umuman sstema turl nav zarralardan tashkl topgan bo`lsh mumkn. Mustakl komponentlar son deganda muvozanat xolatda sstema xtyory modda mkdordan tashkl topgan bo`lsh mumkn. Kmyovy aylansh tufayl to`lk nav sondan kam bo`lsh mumkn. Umumlashgan termodnamk munosabat (I, II prntsplar) ko`p komponentl xolatlar uchun µdn va Ndµ n µ dn va N dµ ( tartb nomer, µ, N- ularnng kmyovy potentsal va zarralar son) larga almashtrb: du d d + µ dn (..4) Bu asosy termodnamk xolatn xarakterlayd va U (, (N )) dfferentsal formada xarakterstk funktsya xsoblanad unng barcha taby parametrlar ekstensv (x m ) ³èñoblanad va asosy rol o`ynayd. Intensv o`zgaruvch (u) funktsya argumentlar orasda yo`k. U xolda (..) dan kelb chkad: U, a (,,{ N} ) U ( a, a { an} ) Agar a\n, bunda N zarralar to`lk son brga teng bo`lsa, U N (,,{ N} ) NU ( N, N,{ N N} ) nav zarra kontsentratsyas, ( U ) { } funktsya (noll tartbl) n N ntensv kattalk,- br jnsl (,,{ N }) ( a, a { }), an kolgan ntensv kattalklar xam shu tarka anklanad. Br komponentl sstema uchun:
36 U + µ N (..5) (..5) n dfferentsallab va (..4) n ayrb Gbbs-Dyugel` munosabatn xosl klamz: d d + N dµ 0 (..6) Bu ntensv o`zgaruvchlar:, R, µ orasdag boxlanshn xarakterlayd. d0 deb (..6) dan br komponentl xol uchun: µ n (..7) Bu esa, zotermk kvazstatsonar jarayon uchun µ p µ o`tsh mkonn bldrad. Ma`lumk, erkn energya F U- dan (..4) shunday ko`rnshn olad: df d d + µ dn (..8) Bu xarakterstk funktsya dfferentsaln anklayd. F (,, {N}) unng ekstensv o`zgaruvchlar {x n } ³èsoblanad., {N }, ntensv o`zgaruvchlar esa, u, xsoblanad. (..) ga ko`ra: F a, (..8) ga asosan (..3) n yozamz: (,,{ N }) F(, a { }) an F + µ N (..9) Bu esa (..5) nng boshka br ko`rnsh xsoblanad. µolgan ekstensv xarakterstk funktsyalar xam shu tarzda anklanad: Ω U µ N, W U +, G U + Bu funktsyalar uchun (..5) shunday ko`rnshga ega:
37 Ω (..0) + N W µ (..) N G µ (..) Br komponentl xolat uchun: G µn (..3) Kmyovy potentsal br zarraga mos keluvch termodnamk potentsalga teng. (..5), (..8), (..0), (..), (..) lar statstk termodnamka apparat deb atalad. Bu ko`p komponentl sstemalar uchun o`rnl. Katta sstemalar uchun o`rnl kchk sstemalarda ekstensv-ntensv tushunchalar o`z xususyatn yo`kotad. (WF -ental`pya-ssklk-saklagch; GZ termodnamk potentsal, (,,N, λ); F(,,N, λ); F(,,N, λ); G(,,N, λ); (U,,N, λ); Ω(,, µ, λ)... ermodinamik KOEFFIsIeNLAR. OLIROIK JARAYoNLAR.IhKI ENeRGIYa A xajm const bo`lganda chk energya U(,) va srt erkn energyas F (,) orkal anklanad. Ichk energya uchun ( ) ( ) U U,,, o`zgaruvchlarga o`tsak: ( ) ( ) ( ) ( ) U U U U,,,, (.. ) Bunda (..) ga
38 Asosan: (.. ) n yozamz U (..) fodanng o`ng tomonn agar gaz xolat ank bo`lsa topsh mumkn. R tenglamadan foydalanb, ( ) 0 U (..3) UU(), U() boxlanshn topsh uchun, blamzk o`zgarmas xajmda U du δ Q d, hu sababl deal gaz uchun chk energya U ( ) ( ) d Gaz bajargan sh U( Т ) + const (..3) da molekulyar fzka nukta nazardan masofadan molekulalarnng o`zaro ta`sr deal gazda e`tborga olnmayd. (e n 0) hu sababl gaz chk energyas o`zgarshga ta`sr klmayd. e k const. const const jarayon, ssklk sxm o`zgarmas poltropk jarayonda ega bo`lamz. (..) formulaga asosan U δ Q cd, du d + d (..4) va du δq δa δq d d d + d Bundan, (..5) ( )
39 Agar termk xolat tenglamalar p (,) ank bo`lsa, (..5)n ntegrallab poltropk xolat tenglamalarn,; ; parametrlar bo`ycha xosl klamz: ) R\, const bo`lganda (..5) tenglama kuydagcha ko`rnshn olad. d d + ( x ) 0 (..6) С Bunda x poltrop ko`rsatgch deylad va х. (..6) n ntegrallab (,) o`zgaruvchlar uchun poltrop tenglamasn olamz: x const. hunngdek, xîëat tenglamasdan (,) parametrlar uchun poltrop tenglaman yozamz: x const. hunngdek, (,) o`zgaruvchlar uchun: x x const. oltrop jarayonlar ayrm xususy xollarn ko`rb o`tamz: Izobark jarayon:, x0 bo`lsa, natja xosl bo`lad const, \const Gey Lyussak konun xosl bo`lad. Izotermk jarayon: Bu xolda ±, x dan ega bo`lamz: const, const Boyl-Marotta konun. С Adabatk jarayon: Bu erda 0, x γ dan ega bo`lamz γ- const; γ- \ γ const, γ const uasson konun. Izoxork jarayon: Bunda ; x ±, poltrop tenglamasn x - ga ko`tarb, chegara kymatlar ega bo`lamz const, \ const harl` konun. Ank bo`ladk, poltrop darajasdan х ± С С x x х ± (..7) х 0 х х γ х 0 х х γ 0-rasm. - rasm. tekslkda poltrop ko`rsatgch - < x < ; γ < x < va <x< γ ntervalda o`zgarad. > 0; < 0 kymatlarda manfy kymatlar burchaklar chkarsda yotad. ( koordnatlarda) zoterma, adabata xîñl bo`ladgan nukta atrofda yotad.
40 stemaga 0 ssklk berlmayd, const gazga ssklk berlad, u shn kompensatsyalayd. U U (); const, du0, δqδa. Ma`lum oralkda poltrop gaz kengayshda 0 < δq < δa va du δq - δa d < 0 Demak d<0 va δq/δ<0. oltrop tenglamasn tekslgda topamz: tekslkda poltrop koefftsent (/) / n ntegrallab / 0 e 0 exp [( x )( γ ) ( x γ ) R] 0 const, (..7) dan foydalandk. uchun R/(γ-) (6.8)nng grafg - rasmda tasvrlangan xususy xolda x0, x, xγ, x ±, <x <γ. Manfy poltrop manfy ssklk sxm mos kelad. С ( ) Masala: a) o`zgaruvch parametrlar (,), ϕ (,)0; b) o`zgaruvch,r, ϕ (,R) 0 bo`lganda termk deal gaz ssklk sxmn shu jarayonlar uchun anklang: ϕ' ' echm: a) Сϕ С, deal gaz ϕ ( ϕ ' ) ϕ ϕ' ϕ' ' ' b) С С + ; ( ϕ ϕ ) ϕ ϕ + ϕ'.3. MUOZANADAGI IZIMLAR ermodinamikai ReJA:.ermodnamkanng asosy konunlar va uslublar..ermodnamkanng asosy tushunchalar. 3.ermodnamk xolat. Muvozanat xolatda bo`lgan xar kanday makroskopk jsm-termodnamk sstema deb atalad. ermodnamk xolatn anklaydgan asosy parametrlar turlcha bo`lsh mumkn. Masalan: suyuklk va gazlarnng xolatn shunday parametrlar :R (bosm),(xajm),(xarorat); - suyuklk pufag katlamda - α (srt taranglk koefftsent), σ - (plenka yuzas), (absolyut xarorat), l (sterjen xolat uzunlg), σ (kesm yuz), f (chuzuvch kuch), e-yung model orkal anklanad. Agar tashkardan brorta ta`sr bo`lmasa, sstemanng stalgan nuktalarda parametrlar o`zgarshsz kolad. Bunday sstema muvozanatl sstema deylad.
41 Agar sstemada muvozanatlk ta`mnlanmagan bo`lsa, unda makroskopk parametrlar gradent mavjud bo`lad. Bosm, zchlk xarorat maydon potentsal, -bunday xoln muvozanatsz xolat deylad. Muvozanatsz termodnamk xolatdan muvozanatl termodnamk xolatga o`tsh jarayonn relaksatsya jarayon deylad. hu jarayon sodr bo`lsh vaktn - relaksatsya vakt deylad. Muvozanat xolatga kaytsh jarayondag relaksatsya vakt, maksmal vakt, o`rtacha vakt, to`lk vakt xar br parametr uchun xarakterl bo`lb termodnamka chegarasda anklash mkon bo`lmayd, chunk jarayonda molekula va atomlar, elektronlar tomondan-energya, massa, mpul`s, magnt moment, nurlansh energyas sngar parametrlar ko`chsh sodr bo`lad. hu sababl relaksatsya vakt masalas blan fzkavy knetka va boshka bo`lmlar shuxullanad. ermodnamkadan relaksatsya tezlgga nsbatan kamrok tezlkda kechadgan jarayonlar o`rganlad. Unda parametrlar o`zgarsh br-brdan juda fark klad, muvozanat xolatga juda yakn bo`lad. Bunday etarlcha sekn jarayonlarn muvozanatl yok kvazstatsonar deb atalad. hu narsa ankk, barcha real jarayonlar muvozanatsz bo`lb, fakatgna kay vaktlardadr, kam yok ko`prok darajada muvozanatl vazyatga yaknlashad. huns ankk, muvozanatl jarayonda barcha parametrlar gradent nolga teng bo`lad. Bundan ma`lumk, smmetrya kuchlar br-brga teskar yo`nalgan bo`lb yxnds nolga teng, to`xr va teskar yo`nalshga sarflangan vakt orkal anklanad. Muvozanatl termodnamk jarayonda to`xr va teskar yo`nalshda vaktn e`tborga olgan xolda xudd shu xolatlarn takrorlansa, bunday muvozanatl jarayon kaytarml deb atalad..4. MUOZANALI KLAIK A KAN IeMA AIIKAI. tatstk yxnd Muvozanatl statstk mexankada statstk yxnd ma`lum br ansambl orkal xsoblanad. ermodnamk sstemanng xossalarn belglovch statstk summa eng ko`p tarkalgan kk usul kanonk va katta kanonk usul orkal anklanad. Kanonk ansamblda N ta zarradan tashkl topgan kvant sstema uchun statstk yxnd Q N kuydagcha bo`lad: Q Q N exp ( βen ) (.4.) n Bunda β/k-gbbs taksmot parametr, e n -sstema kvant xolatlar energyas, n kvant xolatlar uchun N ta br xl zarralar uchun klassk sstema statstk yxnd Q dггех q 3N N! ( πh) ( βh ( )) N, (.4.)
42 Bunda N (R,q) fazovy fazo uchun Gaml`ton funktsyas ( π ) 3N h Geyzenberg anklg bo`lb. Fazovy fazonng elementar xajmda joylashgan zarralar uchun o`rnldr. /N! Kvant ayn zarralar uchun ko`paytma statstk yxnd blan F erkn energya orasda boxlansh kanonk ansambl uchun Katta kanonk ansambl uchun statstk summa L F-k ln Q (.4.3) Z exp µn N 0 n [ ( )] β E en (.4.4) Bunda µ - kmyovy potentsal, e en sstema energya termodnamk potentsal blan statstk yxnd orasdag boxlansh Ω -k lnl, (.4.5) blan anklanad. tatstk yxnd turl ansambllar uchun Laplas-Melln almashtrsh orkal anklanad. Masalan: xolat zchlg mkrokanonk ansambl statstk yxnds xsoblanad va statstk yxnd Q N, blan kuydagcha boxlanshga ega: u xolda Q 0 ( E) exp( βe) ρ de (.4.6) ρ π σ + ( E) Q( β ) exp( βe) dβ σ (.4.7) N 0 Kanonk ansambl uchun (.4.4) n ( ) N L exp βµ N Q ko`rnshda yozamz (.4.7) formula Laplasnng dskret almashtrlsh deb atalsh mumkn. est masalalar echshda o`rtacha kymat xarakterstkasdan emas balk, statstk summa kymatdan ya`n mkroskopk analogdan foydalansh maksadga muvofk. ermodnamk makroskopk sstemada statstk summan chk energya uchun U < E > lnq β E (.4.8)
43 Katta kanonk ansamblda zarralar uchun o`rtacha son < N > < N > l nz (.4.9) β µ, β.aksmot funktsyas Kanonk ansamblda kvant taksmot funktsyas (zchlk operator) H ρ a exp K (.4.8) blan anklanad. H sstema Gaml`ton operator,qunng statstk yxnds ( H K ) Q pexp. (.4.0) Fzkavy kattalklar o`rtacha kymat ( ρa)( p ) A < A > p ρ (.4.0) bo`lad. Masalan, energetk xolatlar uchun taksmot funktsyas va o`rtacha kymatn anklash formulalar: E exp n ; Q K ω n (.4.) ( ) E < A > A E n n exp Q K. (.4.) Bunda A(e n )-; A operatornng dagonal matrtsas (.4.) va (.4.) formulalar klassk o`xshashlg kuydagcha E ω ( E) exp ; Q K (.4.3) ( E) ω( E) A( ) ρ (.4.4) < A > de E ρ (E) - energetk xolat zchlg. Klassk sstemalarda taksmot funktsyas umumlashgan mpul`slar va koordnata uchun Gamel`ton funktsyas orkal fodalanad. N H ( r, ρ ) + U ( r) (.4.5) m potentsal energya U(ch) fakatgna zarralar koordnatlar orkal anklanad.
44 3N ( r, ρ ) ( π ) n( r) t( ρ ) N ρ h (.4.6) Bunda koordnatalar bo`ycha taksmot funktsyas n Z- konfguratson ntegral ( r) Z exp[ U ( r) K ] (.4.7) ( r) U Z dr exp N! K Br kymatl funktsya uchun mpul`slar bo`ycha taksmot (.4.8) f ( ρ ) exp 3/ ( πmk ) mk (.4.8) f(ρ) funktsya uchta funktsyadan tashkl topganlgn xsobga olb : f x x y z x exp / ( πmk ) mk ( ρ ) f ( ) f ( ) f ( ); f ( ) Keltrlgan funktsyalar orkal fzkavy kattalklarnng fakatgna umumlashgan koordnatalar va mpul`slarga boxlk bo`lgan o`rtacha kymatlar <A> d r n (r) A (r) (.4.0) va <B> dpf(p)b(p) (.4.) formulalar orkal anklanad. stemadag zarralarnng tashk maydon blan o`zaro boxlansh energyas U N ( r) U ( r) (.4.) ga teng. n(r) funktsya mpul`slar bo`ycha taksmot funktsyasga o`xshashdr. Ayrm zarralar uchun taksmot funktsyas n N ( r) n( r ) (.4.3) Normallash shart ( r) drn (.4.4) bo`lad. ermodnamk kattalklar o`rtacha kymatn anklashda taksmot funktsyalardan foydalanshn test masalalar tuzsh va echsh msolda ko`rb o`taylk: est masalalarn echsh namunalar: Masala. Kanonk ansamblda statstk summadan foydalanb, br atoml deal gaz uchun xolat chk energyasn va ssklk sxm tenglamalarn anklang. A). Br atoml N zarral deal gaz uchun Gaml`ton funktsyasn belglang.
45 A N. H ( q, p) Б. H ( q, p) m m В. H m Г H. m B). rt erkn energyasn anklang Д. H 3m 3 А. F KNl n + K + B Б. F KN N 3 В. F KNln Г. F KN K N 3 Д. F KN ln ). Issklk sxmn belglang. 3 A. R Б. KN В. Г. KN Д. KN 3 D). Bosmn anklang. F KN A. Б. NK В. Г. KN Д. KN / N
46
funksiyaning birinchi tartibli xususiy hosilasidan
 A RUZA 8 URAKKA UNKSIYANING HOSILASI. TO`LA DIЕRЕNTSIAL TUSHUNCHASI. EKSTRЕULARI. TAQRIIY HISOLASH. DASTURIY PAKETLAR YORDAIDA HISOLASH. aqsad: Talabalarga ko po zgaruvchl uksalarg deresal, ekstremumlar
A RUZA 8 URAKKA UNKSIYANING HOSILASI. TO`LA DIЕRЕNTSIAL TUSHUNCHASI. EKSTRЕULARI. TAQRIIY HISOLASH. DASTURIY PAKETLAR YORDAIDA HISOLASH. aqsad: Talabalarga ko po zgaruvchl uksalarg deresal, ekstremumlar
O ZBEKISTON RESPUBLIKASI OLIY VA O RTA MAXSUS TA LIM VAZIRLIGI ALISHER NAVOIY NOMIDAGI SAMARQAND DAVLAT UNIVERSITETI
 O ZBEKISTON RESPUBLIKSI OLIY V O RT MXSUS T LIM VZIRLIGI LISHER NVOIY NOMIDGI SMRQND DVLT UNIVERSITETI XBOROTLSHTIRISH TEXNOLOGIYLRI KFEDRSI «NZRIY MEXNIK» fandan o quv-usluby M J M U Matematka va meanka
O ZBEKISTON RESPUBLIKSI OLIY V O RT MXSUS T LIM VZIRLIGI LISHER NVOIY NOMIDGI SMRQND DVLT UNIVERSITETI XBOROTLSHTIRISH TEXNOLOGIYLRI KFEDRSI «NZRIY MEXNIK» fandan o quv-usluby M J M U Matematka va meanka
Ehtimollar nazariyasi va matematik statistika
 O ZBEKISTON RESPUBLIKASI OLIY VA O RTA MAXSUS TA LIM VAZIRLIGI Toshket Molya Isttut E. Mamurov T. Adrov Ehtmollar azaryas va matematk statstka o quv qo llama Toshket-005 E. Mamurov, T. Adrov. Ehtmollar
O ZBEKISTON RESPUBLIKASI OLIY VA O RTA MAXSUS TA LIM VAZIRLIGI Toshket Molya Isttut E. Mamurov T. Adrov Ehtmollar azaryas va matematk statstka o quv qo llama Toshket-005 E. Mamurov, T. Adrov. Ehtmollar
OLIY MATEMATIKA. Ehtimollar nazariyasi va matematik statistika bo yicha mustaqil ishlarni bajarish uchun qo llanma
 O ZBEКISTON RESPUBLIКASI OLIY VA O RTA MAXSUS TA LIM VAZIRLIGI Abu Rayho Beruy omdag TOSHКENT DAVLAT TEXNIКA UNIVERSITETI OLIY MATEMATIKA Ehtmollar azaryas va matematk statstka bo ycha mustaql shlar bajarsh
O ZBEКISTON RESPUBLIКASI OLIY VA O RTA MAXSUS TA LIM VAZIRLIGI Abu Rayho Beruy omdag TOSHКENT DAVLAT TEXNIКA UNIVERSITETI OLIY MATEMATIKA Ehtmollar azaryas va matematk statstka bo ycha mustaql shlar bajarsh
Parts Manual. Trio Mobile Surgery Platform. Model 1033
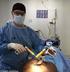 Trio Mobile Surgery Platform Model 1033 Parts Manual For parts or technical assistance: Pour pièces de service ou assistance technique : Für Teile oder technische Unterstützung Anruf: Voor delen of technische
Trio Mobile Surgery Platform Model 1033 Parts Manual For parts or technical assistance: Pour pièces de service ou assistance technique : Für Teile oder technische Unterstützung Anruf: Voor delen of technische
YARIMO TKAZGICHLARDA ULTRATOVUSHNING YUTILISHI VA KUCHAYISHI HAQIDA
 O ZBEKISTON RESPUBLIKASI OLIY VA O RTA AXSUS TA LI VAZIRLIGI SAARQAND DAVLAT UNIVERSITETI Qo l yozma huquqa UDK 537.3.33.534.8 URINOV JASHID ORTIQOVICH YARIO TKAZGICHLARDA ULTRATOVUSHNING YUTILISHI VA
O ZBEKISTON RESPUBLIKASI OLIY VA O RTA AXSUS TA LI VAZIRLIGI SAARQAND DAVLAT UNIVERSITETI Qo l yozma huquqa UDK 537.3.33.534.8 URINOV JASHID ORTIQOVICH YARIO TKAZGICHLARDA ULTRATOVUSHNING YUTILISHI VA
O zbekiston Respublikasi oliy va o rta maxsus ta lim vazirligi. Toshkent moliya instituti. Q Safaeva. Matematik dasturlash.
 O zbeksto Respublkas oly va o rta asus ta l vazrlg Toshket olya sttut Q Safaeva. Mateatk dasturlash (Darslk) Toshket. Q.Safaeva. Mateatk dasturlash. Darslk TMI-y. Aotatsya: Ushbu ktob ateatk dasturlashda
O zbeksto Respublkas oly va o rta asus ta l vazrlg Toshket olya sttut Q Safaeva. Mateatk dasturlash (Darslk) Toshket. Q.Safaeva. Mateatk dasturlash. Darslk TMI-y. Aotatsya: Ushbu ktob ateatk dasturlashda
(... )..!, ".. (! ) # - $ % % $ & % 2007
 (! ), "! ( ) # $ % & % $ % 007 500 ' 67905:5394!33 : (! ) $, -, * +,'; ), -, *! ' - " #!, $ & % $ ( % %): /!, " ; - : - +', 007 5 ISBN 978-5-7596-0766-3 % % - $, $ &- % $ % %, * $ % - % % # $ $,, % % #-
(! ), "! ( ) # $ % & % $ % 007 500 ' 67905:5394!33 : (! ) $, -, * +,'; ), -, *! ' - " #!, $ & % $ ( % %): /!, " ; - : - +', 007 5 ISBN 978-5-7596-0766-3 % % - $, $ &- % $ % %, * $ % - % % # $ $,, % % #-
Υπολογισμός & Πρόρρηση. Θερμοδυναμικών Ιδιοτήτων
 Υπολογισμός & Πρόρρηση Θερμοδυναμικών Ιδιοτήτων d du d Θερμοδυναμικές Ιδιότητες d dh d d d du d d dh U A H G d d da d d dg d du dq dq d / d du dq Θεμελιώδεις Συναρτήσεις περιέχουν όλες τις πληροφορίες
Υπολογισμός & Πρόρρηση Θερμοδυναμικών Ιδιοτήτων d du d Θερμοδυναμικές Ιδιότητες d dh d d d du d d dh U A H G d d da d d dg d du dq dq d / d du dq Θεμελιώδεις Συναρτήσεις περιέχουν όλες τις πληροφορίες
O ZBEKISTON RESPUBLIKASI OLIY VA O RTA MAXSUS TA LIM VAZIRLIGI QARSHI MUHANDISLIK IQTISODIYOT INSTITUTI ENERGETIKA FAKULTETI
 O ZBEKISTON RESPUBLIKASI OLIY VA O RTA MAXSUS TA LIM VAZIRLIGI QARSHI MUHANDISLIK IQTISODIYOT INSTITUTI ENERGETIKA FAKULTETI «Muqobil energiya manbalari» ta lim yo nalishi 195-guruhi talabasi Rahmatov
O ZBEKISTON RESPUBLIKASI OLIY VA O RTA MAXSUS TA LIM VAZIRLIGI QARSHI MUHANDISLIK IQTISODIYOT INSTITUTI ENERGETIKA FAKULTETI «Muqobil energiya manbalari» ta lim yo nalishi 195-guruhi talabasi Rahmatov
 μ μ dω I ν S da cos θ da λ λ Γ α/β MJ Capítulo 1 % βpic ɛ Eridani V ega β P ic F ormalhaut 10 9 15% 70 Virgem 47 Ursa Maior Debris Disk Debris Disk μ 90% L ac = GM M ac R L ac R M M ac L J T
μ μ dω I ν S da cos θ da λ λ Γ α/β MJ Capítulo 1 % βpic ɛ Eridani V ega β P ic F ormalhaut 10 9 15% 70 Virgem 47 Ursa Maior Debris Disk Debris Disk μ 90% L ac = GM M ac R L ac R M M ac L J T
... 5 A.. RS-232C ( ) RS-232C ( ) RS-232C-LK & RS-232C-MK RS-232C-JK & RS-232C-KK
 RS-3C WIWM050 014.1.9 P1 :8... 1... 014.0.1 1 A... 014.0. 1... RS-3C()...01.08.03 A.. RS-3C()...01.08.03 3... RS-3C()... 003.11.5 4... RS-3C ()... 00.10.01 5... RS-3C().008.07.16 5 A.. RS-3C().0 1.08.
RS-3C WIWM050 014.1.9 P1 :8... 1... 014.0.1 1 A... 014.0. 1... RS-3C()...01.08.03 A.. RS-3C()...01.08.03 3... RS-3C()... 003.11.5 4... RS-3C ()... 00.10.01 5... RS-3C().008.07.16 5 A.. RS-3C().0 1.08.
ELEKTRODINAMIKA fanidan
 O zbekiston Respublikasi Oliy va o rta maxsus ta lim vazirligi Z.M.Bobur nomidagi Andijon davlat universiteti FIZIKA kafedrasi ELEKTRODINAMIKA fanidan ma ruza matnlari Tuzuvchi: dots M.Nosirov Andijon-06
O zbekiston Respublikasi Oliy va o rta maxsus ta lim vazirligi Z.M.Bobur nomidagi Andijon davlat universiteti FIZIKA kafedrasi ELEKTRODINAMIKA fanidan ma ruza matnlari Tuzuvchi: dots M.Nosirov Andijon-06
Π Ο Λ Ι Τ Ι Κ Α Κ Α Ι Σ Τ Ρ Α Τ Ι Ω Τ Ι Κ Α Γ Ε Γ Ο Ν Ο Τ Α
 Α Ρ Χ Α Ι Α Ι Σ Τ Ο Ρ Ι Α Π Ο Λ Ι Τ Ι Κ Α Κ Α Ι Σ Τ Ρ Α Τ Ι Ω Τ Ι Κ Α Γ Ε Γ Ο Ν Ο Τ Α Σ η µ ε ί ω σ η : σ υ ν ά δ ε λ φ ο ι, ν α µ ο υ σ υ γ χ ω ρ ή σ ε τ ε τ ο γ ρ ή γ ο ρ ο κ α ι α τ η µ έ λ η τ ο ύ
Α Ρ Χ Α Ι Α Ι Σ Τ Ο Ρ Ι Α Π Ο Λ Ι Τ Ι Κ Α Κ Α Ι Σ Τ Ρ Α Τ Ι Ω Τ Ι Κ Α Γ Ε Γ Ο Ν Ο Τ Α Σ η µ ε ί ω σ η : σ υ ν ά δ ε λ φ ο ι, ν α µ ο υ σ υ γ χ ω ρ ή σ ε τ ε τ ο γ ρ ή γ ο ρ ο κ α ι α τ η µ έ λ η τ ο ύ
#%" )*& ##+," $ -,!./" %#/%0! %,!
 -!"#$% -&!'"$ & #("$$, #%" )*& ##+," $ -,!./" %#/%0! %,! %!$"#" %!#0&!/" /+#0& 0.00.04. - 3 3,43 5 -, 4 $ $.. 04 ... 3. 6... 6.. #3 7 8... 6.. %9: 3 3 7....3. % 44 8... 6.4. 37; 3,, 443 8... 8.5. $; 3
-!"#$% -&!'"$ & #("$$, #%" )*& ##+," $ -,!./" %#/%0! %,! %!$"#" %!#0&!/" /+#0& 0.00.04. - 3 3,43 5 -, 4 $ $.. 04 ... 3. 6... 6.. #3 7 8... 6.. %9: 3 3 7....3. % 44 8... 6.4. 37; 3,, 443 8... 8.5. $; 3
BITIRUV MALAKAVIY ISHI
 O'ZBEKISTON ESPUBLIKASI OLIY VA O'TA AXSUS TA'LI VAZILIGI ALISHE NAVOIY NOIDAGI SAAQAND DAVLAT UNIVESITETI EXANIKA ATEATIKA FAKULTETI atematk fka a fksoal aal kafedas ade Olmos 5 - matematka ta'lm o'alsh
O'ZBEKISTON ESPUBLIKASI OLIY VA O'TA AXSUS TA'LI VAZILIGI ALISHE NAVOIY NOIDAGI SAAQAND DAVLAT UNIVESITETI EXANIKA ATEATIKA FAKULTETI atematk fka a fksoal aal kafedas ade Olmos 5 - matematka ta'lm o'alsh
 Molekulare Ebene (biochemische Messungen) Zelluläre Ebene (Elektrophysiologie, Imaging-Verfahren) Netzwerk Ebene (Multielektrodensysteme) Areale (MRT, EEG...) Gene Neuronen Synaptische Kopplung kleine
Molekulare Ebene (biochemische Messungen) Zelluläre Ebene (Elektrophysiologie, Imaging-Verfahren) Netzwerk Ebene (Multielektrodensysteme) Areale (MRT, EEG...) Gene Neuronen Synaptische Kopplung kleine
Κλασική και στατιστική Θερμοδυναμική
 ΠΑΝΕΠΙΣΤΗΜΙΟ ΙΩΑΝΝΙΝΩΝ ΑΝΟΙΚΤΑ ΑΚΑΔΗΜΑΪΚΑ ΜΑΘΗΜΑΤΑ Κλασική και στατιστική Θερμοδυναμική Σύνοψη Κλασικής Θερμοδυναμικής Διδάσκων: Καθηητής Ιωάννης Παναιωτόπουλος Άδειες Χρήσης Το παρόν εκπαιδευτικό υλικό
ΠΑΝΕΠΙΣΤΗΜΙΟ ΙΩΑΝΝΙΝΩΝ ΑΝΟΙΚΤΑ ΑΚΑΔΗΜΑΪΚΑ ΜΑΘΗΜΑΤΑ Κλασική και στατιστική Θερμοδυναμική Σύνοψη Κλασικής Θερμοδυναμικής Διδάσκων: Καθηητής Ιωάννης Παναιωτόπουλος Άδειες Χρήσης Το παρόν εκπαιδευτικό υλικό
C M. V n: n =, (D): V 0,M : V M P = ρ ρ V V. = ρ
 »»...» -300-0 () -300-03 () -3300 3.. 008 4 54. 4. 5 :.. ;.. «....... :. : 008. 37.. :....... 008.. :. :.... 54. 4. 5 5 6 ... : : 3 V mnu V mn AU 3 m () ; N (); N A 6030 3 ; ( ); V 3. : () 0 () 0 3 ()
»»...» -300-0 () -300-03 () -3300 3.. 008 4 54. 4. 5 :.. ;.. «....... :. : 008. 37.. :....... 008.. :. :.... 54. 4. 5 5 6 ... : : 3 V mnu V mn AU 3 m () ; N (); N A 6030 3 ; ( ); V 3. : () 0 () 0 3 ()
Dozent: Alexander Shnirman Institut für Theorie der Kondensierten Materie
 Sommer-Semester 20 Moderne Theoretische Physik III Statistische Physik Dozent: Alexander Shnirman Institut für Theorie der Kondensierten Materie Di 09:45-:5, Lehmann HS 022, Geb 30.22 Do 09:45-:5, Lehmann
Sommer-Semester 20 Moderne Theoretische Physik III Statistische Physik Dozent: Alexander Shnirman Institut für Theorie der Kondensierten Materie Di 09:45-:5, Lehmann HS 022, Geb 30.22 Do 09:45-:5, Lehmann
Teor imov r. ta matem. statist. Vip. 94, 2016, stor
 eor imov r. ta matem. statist. Vip. 94, 6, stor. 93 5 Abstract. e article is devoted to models of financial markets wit stocastic volatility, wic is defined by a functional of Ornstein-Ulenbeck process
eor imov r. ta matem. statist. Vip. 94, 6, stor. 93 5 Abstract. e article is devoted to models of financial markets wit stocastic volatility, wic is defined by a functional of Ornstein-Ulenbeck process
VIII. TEST. bayon etish usullarini ifodalovchi zamonaviy nazariya; bayon etish usullarini ifodalovchi zamonaviy nazariya;
 VIII. TEST 1. Atom fizikasi: +Atom va u bilan bog lik hodisalar fizikasini o rganuvchi fan; - Atom yadrosini tuzilishi xossalari va bir - biriga aylanishlarini o rganadi; - mikrozarrachalar va ulardan
VIII. TEST 1. Atom fizikasi: +Atom va u bilan bog lik hodisalar fizikasini o rganuvchi fan; - Atom yadrosini tuzilishi xossalari va bir - biriga aylanishlarini o rganadi; - mikrozarrachalar va ulardan
: Ω F F 0 t T P F 0 t T F 0 P Q. Merton 1974 XT T X T XT. T t. V t t X d T = XT [V t/t ]. τ 0 < τ < X d T = XT I {V τ T } δt XT I {V τ<t } I A
![: Ω F F 0 t T P F 0 t T F 0 P Q. Merton 1974 XT T X T XT. T t. V t t X d T = XT [V t/t ]. τ 0 < τ < X d T = XT I {V τ T } δt XT I {V τ<t } I A : Ω F F 0 t T P F 0 t T F 0 P Q. Merton 1974 XT T X T XT. T t. V t t X d T = XT [V t/t ]. τ 0 < τ < X d T = XT I {V τ T } δt XT I {V τ<t } I A](/thumbs/91/105433318.jpg) 2012 4 Chinese Journal of Applied Probability and Statistics Vol.28 No.2 Apr. 2012 730000. :. : O211.9. 1..... Johnson Stulz [3] 1987. Merton 1974 Johnson Stulz 1987. Hull White 1995 Klein 1996 2008 Klein
2012 4 Chinese Journal of Applied Probability and Statistics Vol.28 No.2 Apr. 2012 730000. :. : O211.9. 1..... Johnson Stulz [3] 1987. Merton 1974 Johnson Stulz 1987. Hull White 1995 Klein 1996 2008 Klein
692.66:
 1 69.66:6-83 05.05.05 -,, 015 .. 7... 8 1.... 19 1.1.,.. 19 1.. 8 1.3.. 1.4... 1.4.1.... 33 36 40 1.4.. 44 1.4.3. -... 48.. 53.,.. 56.1., -....... 56..... 6.3.... 71.. 76 3.,.... 77 3 3.1.... 77 3.1.1....
1 69.66:6-83 05.05.05 -,, 015 .. 7... 8 1.... 19 1.1.,.. 19 1.. 8 1.3.. 1.4... 1.4.1.... 33 36 40 1.4.. 44 1.4.3. -... 48.. 53.,.. 56.1., -....... 56..... 6.3.... 71.. 76 3.,.... 77 3 3.1.... 77 3.1.1....
 !"#!"!"# $ "# '()!* '+!*, -"*!" $ "#. /01 023 43 56789:3 4 ;8< = 7 >/? 44= 7 @ 90A 98BB8: ;4B0C BD :0 E D:84F3 B8: ;4BG H ;8
!"#!"!"# $ "# '()!* '+!*, -"*!" $ "#. /01 023 43 56789:3 4 ;8< = 7 >/? 44= 7 @ 90A 98BB8: ;4B0C BD :0 E D:84F3 B8: ;4BG H ;8
m i N 1 F i = j i F ij + F x
 N m i i = 1,..., N m i Fi x N 1 F ij, j = 1, 2,... i 1, i + 1,..., N m i F i = j i F ij + F x i mi Fi j Fj i mj O P i = F i = j i F ij + F x i, i = 1,..., N P = i F i = N F ij + i j i N i F x i, i = 1,...,
N m i i = 1,..., N m i Fi x N 1 F ij, j = 1, 2,... i 1, i + 1,..., N m i F i = j i F ij + F x i mi Fi j Fj i mj O P i = F i = j i F ij + F x i, i = 1,..., N P = i F i = N F ij + i j i N i F x i, i = 1,...,
o quv yili matematikadan 9-sinf imtihon biletlari yechimlari 1-bilet = 0,75 1,2+0,9. = 73; Javob: <CAB= 730
 . (,,87),+0,9 40: 50. + x+ X, 8±0 ; x 6 8 0 6 05-06-o quv yili matematikadan 9-sinf imtihon biletlari yechimlari -bilet 0,75,+0,9 90 0,9+0,9 90 0; ; (x-) +(x+),5(x-)(x+); x 4x-4+4x+43x -3; 3x -8x-30; (-8)
. (,,87),+0,9 40: 50. + x+ X, 8±0 ; x 6 8 0 6 05-06-o quv yili matematikadan 9-sinf imtihon biletlari yechimlari -bilet 0,75,+0,9 90 0,9+0,9 90 0; ; (x-) +(x+),5(x-)(x+); x 4x-4+4x+43x -3; 3x -8x-30; (-8)
2.60 ακαριαία. σιγά σιγά
 ΑΣΚΗΣΕΙΣ .60 Θερμικά μονωμένος κύλινδρος χωρίζεται σε δύο μέρη από αδιαβατικό, αβαρές έμβολο που κινείται χωρίς τριβή. Αρχικά το έμβολο συγκρατείται ακίνητο. Ο κύλινδρος περιέχει n mles ιδανικού αερίου
ΑΣΚΗΣΕΙΣ .60 Θερμικά μονωμένος κύλινδρος χωρίζεται σε δύο μέρη από αδιαβατικό, αβαρές έμβολο που κινείται χωρίς τριβή. Αρχικά το έμβολο συγκρατείται ακίνητο. Ο κύλινδρος περιέχει n mles ιδανικού αερίου
Fizika fanidan test topshiriqlarini yechish bo yicha abituriyentlar uchun ayrim tavsiyalar
 Fizika fanidan test topshiriqlarini yechish bo yicha abituriyentlar uchun ayrim tavsiyalar Quyida fizika fanidan test topshiriqlarini bajarishga doir bir necha uslubiy tavsiyalarga beriladi. - test topshirig
Fizika fanidan test topshiriqlarini yechish bo yicha abituriyentlar uchun ayrim tavsiyalar Quyida fizika fanidan test topshiriqlarini bajarishga doir bir necha uslubiy tavsiyalarga beriladi. - test topshirig
TALAR ROSA -. / ',)45$%"67789
 TALAR ROSA!"#"$"%$&'$%(" )*"+%(""%$," *$ -. / 0"$%%"$&'1)2$3!"$ ',)45$%"67789 ," %"(%:,;,"%,$"$)$*2
TALAR ROSA!"#"$"%$&'$%(" )*"+%(""%$," *$ -. / 0"$%%"$&'1)2$3!"$ ',)45$%"67789 ," %"(%:,;,"%,$"$)$*2
 γ 1 6 M = 0.05 F M = 0.05 F M = 0.2 F M = 0.2 F M = 0.05 F M = 0.05 F M = 0.05 F M = 0.2 F M = 0.05 F 2 2 λ τ M = 6000 M = 10000 M = 15000 M = 6000 M = 10000 M = 15000 1 6 τ = 36 1 6 τ = 102 1 6 M = 5000
γ 1 6 M = 0.05 F M = 0.05 F M = 0.2 F M = 0.2 F M = 0.05 F M = 0.05 F M = 0.05 F M = 0.2 F M = 0.05 F 2 2 λ τ M = 6000 M = 10000 M = 15000 M = 6000 M = 10000 M = 15000 1 6 τ = 36 1 6 τ = 102 1 6 M = 5000
OQIM TERMODINAMIKASI. Reja: 1. Asosiy tushunchalar. 2. Bajariladigan ish. Oqim uchun termodinamikaning birinchi qonuni tenglamasi. 3.
 OQIM TERMODINAMIKASI Reja:. Asosiy tushunchaar.. Bajariadigan ish. Oqim uchun termodinamikaning birinchi qonuni tengamasi. 3. Drosseash. Asosiy tushunchaar Bugʻ va gaz turbinaari, turbokompressorar, reaktiv
OQIM TERMODINAMIKASI Reja:. Asosiy tushunchaar.. Bajariadigan ish. Oqim uchun termodinamikaning birinchi qonuni tengamasi. 3. Drosseash. Asosiy tushunchaar Bugʻ va gaz turbinaari, turbokompressorar, reaktiv
θβ1.0γθμθ81.β0 (07η.8) - - -, , 2015
 - Ч Ч Ы - 05 θβ.0γθμθ8.β0 (07η.8) μ.. (. 3, 4),.. (. 3, 4),.. (. 4),.. (. 3), Е.. (. 3),.. я (. 3, 4),.. я (. 4), Е.. я (. 4),.. (. 3),.. (. ),.. Ф (. )..:. /......μ -. -, 05. 78., «-»,, «-». μ -,, -,.,
- Ч Ч Ы - 05 θβ.0γθμθ8.β0 (07η.8) μ.. (. 3, 4),.. (. 3, 4),.. (. 4),.. (. 3), Е.. (. 3),.. я (. 3, 4),.. я (. 4), Е.. я (. 4),.. (. 3),.. (. ),.. Ф (. )..:. /......μ -. -, 05. 78., «-»,, «-». μ -,, -,.,
!"#$ % &# &%#'()(! $ * +
 ,!"#$ % &# &%#'()(! $ * + ,!"#$ % &# &%#'()(! $ * + 6 7 57 : - - / :!", # $ % & :'!(), 5 ( -, * + :! ",, # $ %, ) #, '(#,!# $$,',#-, 4 "- /,#-," -$ '# &",,#- "-&)'#45)')6 5! 6 5 4 "- /,#-7 ",',8##! -#9,!"))
,!"#$ % &# &%#'()(! $ * + ,!"#$ % &# &%#'()(! $ * + 6 7 57 : - - / :!", # $ % & :'!(), 5 ( -, * + :! ",, # $ %, ) #, '(#,!# $$,',#-, 4 "- /,#-," -$ '# &",,#- "-&)'#45)')6 5! 6 5 4 "- /,#-7 ",',8##! -#9,!"))
 W τ R W j N H = 2 F obj b q N F aug F obj b q Ψ F aug Ψ ( ) ϱ t + + p = 0 = 0 Ω f = Γ Γ b ϱ = (, t) = (, t) Ω f Γ b ( ) ϱ t + + p = V max 4 3 2 1 0-1 -2-3 -4-4 -3-2 -1 0 1 2 3 4 x 4 x 1 V mn V max
W τ R W j N H = 2 F obj b q N F aug F obj b q Ψ F aug Ψ ( ) ϱ t + + p = 0 = 0 Ω f = Γ Γ b ϱ = (, t) = (, t) Ω f Γ b ( ) ϱ t + + p = V max 4 3 2 1 0-1 -2-3 -4-4 -3-2 -1 0 1 2 3 4 x 4 x 1 V mn V max
Stereometriya asoslari. 8. Aksiomatik nazariya. Stereometriya aksiomalari. Ularning planimetriya aksiomalari bilan aloqasi. Fazodagi aksiomalar
 Stereometriya asoslari. 8. Aksiomatik nazariya. Stereometriya aksiomalari. Ularning planimetriya aksiomalari bilan aloqasi. Fazodagi aksiomalar Stereometriya, ya'ni fazodagi geometriyani o'rganishni biz
Stereometriya asoslari. 8. Aksiomatik nazariya. Stereometriya aksiomalari. Ularning planimetriya aksiomalari bilan aloqasi. Fazodagi aksiomalar Stereometriya, ya'ni fazodagi geometriyani o'rganishni biz
ITU-R P (2012/02) &' (
 ITU-R P.530-4 (0/0) $ % " "#! &' ( P ITU-R P. 530-4 ii.. (IPR) (ITU-T/ITU-R/ISO/IEC).ITU-R http://www.itu.int/itu-r/go/patents/en. ITU-T/ITU-R/ISO/IEC (http://www.itu.int/publ/r-rec/en ) () ( ) BO BR BS
ITU-R P.530-4 (0/0) $ % " "#! &' ( P ITU-R P. 530-4 ii.. (IPR) (ITU-T/ITU-R/ISO/IEC).ITU-R http://www.itu.int/itu-r/go/patents/en. ITU-T/ITU-R/ISO/IEC (http://www.itu.int/publ/r-rec/en ) () ( ) BO BR BS
http://www.mathematica.gr/forum/viewtopic.php?f=109&t=15584
 Επιμέλεια : xr.tsif Σελίδα 1 ΠΡΟΤΕΙΝΟΜΕΝΕΣ ΑΣΚΗΣΕΙΣ ΓΙΑ ΜΑΘΗΤΙΚΟΥΣ ΔΙΑΓΩΝΙΣΜΟΥΣ ΕΚΦΩΝΗΣΕΙΣ ΤΕΥΧΟΣ ΑΣΚΗΣΕΙΣ 101-00 Αφιερωμέν σε κάθε μαθητή πυ ασχλείται ή πρόκειται να ασχληθεί με Μαθηματικύς διαγωνισμύς
Επιμέλεια : xr.tsif Σελίδα 1 ΠΡΟΤΕΙΝΟΜΕΝΕΣ ΑΣΚΗΣΕΙΣ ΓΙΑ ΜΑΘΗΤΙΚΟΥΣ ΔΙΑΓΩΝΙΣΜΟΥΣ ΕΚΦΩΝΗΣΕΙΣ ΤΕΥΧΟΣ ΑΣΚΗΣΕΙΣ 101-00 Αφιερωμέν σε κάθε μαθητή πυ ασχλείται ή πρόκειται να ασχληθεί με Μαθηματικύς διαγωνισμύς
Errata (Includes critical corrections only for the 1 st & 2 nd reprint)
 Wedesday, May 5, 3 Erraa (Icludes criical correcios oly for he s & d repri) Advaced Egieerig Mahemaics, 7e Peer V O eil ISB: 978474 Page # Descripio 38 ie 4: chage "w v a v " "w v a v " 46 ie : chage "y
Wedesday, May 5, 3 Erraa (Icludes criical correcios oly for he s & d repri) Advaced Egieerig Mahemaics, 7e Peer V O eil ISB: 978474 Page # Descripio 38 ie 4: chage "w v a v " "w v a v " 46 ie : chage "y
 Z = 1.2 X 1 + 1, 4 X 2 + 3, 3 X 3 + 0, 6 X 4 + 0, 999 X 5. X 1 X 2 X 2 X 3 X 4 X 4 X 5 X 4 X 4 Z = 0.717 X 1 + 0.847 X 2 + 3.107 X 3 + 0.420 X 4 + 0.998 X 5. X 5 X 4 Z = 6.56 X 1 + 3.26 X 2 + 6.72 X 3
Z = 1.2 X 1 + 1, 4 X 2 + 3, 3 X 3 + 0, 6 X 4 + 0, 999 X 5. X 1 X 2 X 2 X 3 X 4 X 4 X 5 X 4 X 4 Z = 0.717 X 1 + 0.847 X 2 + 3.107 X 3 + 0.420 X 4 + 0.998 X 5. X 5 X 4 Z = 6.56 X 1 + 3.26 X 2 + 6.72 X 3
OPTIKA. YORUG`LIKNING TABIATI 1. Yorug`likning tabiati. Yorug`lik to`lqinlarining monoxromatikligi va kogerentligi. 2. Fotometrik kattaliklar. 3.
 OPTIKA. YORUG`LIKNING TABIATI 1. Yorug`likning tabiati. Yorug`lik to`lqinlarining monoxromatikligi va kogerentligi. 2. Fotometrik kattaliklar. 3. Yorug`lik interferensiyasi. 4. Ikki nurdan kuzatiladigan
OPTIKA. YORUG`LIKNING TABIATI 1. Yorug`likning tabiati. Yorug`lik to`lqinlarining monoxromatikligi va kogerentligi. 2. Fotometrik kattaliklar. 3. Yorug`lik interferensiyasi. 4. Ikki nurdan kuzatiladigan
Α Ρ Ι Θ Μ Ο Σ : 6.913
 Α Ρ Ι Θ Μ Ο Σ : 6.913 ΠΡΑΞΗ ΚΑΤΑΘΕΣΗΣ ΟΡΩΝ ΔΙΑΓΩΝΙΣΜΟΥ Σ τ η ν Π ά τ ρ α σ ή μ ε ρ α σ τ ι ς δ ε κ α τ έ σ σ ε ρ ι ς ( 1 4 ) τ ο υ μ ή ν α Ο κ τ ω β ρ ί ο υ, η μ έ ρ α Τ ε τ ά ρ τ η, τ ο υ έ τ ο υ ς δ
Α Ρ Ι Θ Μ Ο Σ : 6.913 ΠΡΑΞΗ ΚΑΤΑΘΕΣΗΣ ΟΡΩΝ ΔΙΑΓΩΝΙΣΜΟΥ Σ τ η ν Π ά τ ρ α σ ή μ ε ρ α σ τ ι ς δ ε κ α τ έ σ σ ε ρ ι ς ( 1 4 ) τ ο υ μ ή ν α Ο κ τ ω β ρ ί ο υ, η μ έ ρ α Τ ε τ ά ρ τ η, τ ο υ έ τ ο υ ς δ
TOSHKENT IRRIGATSIYA VA MELIORATSIYA INSTITUTI BUXORO FILIALI "UMUMKASBIY FANLAR" KAFEDRASI "CHIZMA GEOMETRIYA VA MUHANDISLIK GRAFIKASI"
 TOSHKENT IRRIGATSIYA VA MELIORATSIYA INSTITUTI BUXORO FILIALI "UMUMKASBIY FANLAR" KAFEDRASI "CHIZMA GEOMETRIYA VA MUHANDISLIK GRAFIKASI" fanidan ma'ruzalar matni Tuzuvchilar: S.R.Djuraeva Buxoro 2016 1
TOSHKENT IRRIGATSIYA VA MELIORATSIYA INSTITUTI BUXORO FILIALI "UMUMKASBIY FANLAR" KAFEDRASI "CHIZMA GEOMETRIYA VA MUHANDISLIK GRAFIKASI" fanidan ma'ruzalar matni Tuzuvchilar: S.R.Djuraeva Buxoro 2016 1
..,..,.. ! " # $ % #! & %
 ..,..,.. - -, - 2008 378.146(075.8) -481.28 73 69 69.. - : /..,..,... : - -, 2008. 204. ISBN 5-98298-269-5. - -,, -.,,, -., -. - «- -»,. 378.146(075.8) -481.28 73 -,..,.. ISBN 5-98298-269-5..,..,.., 2008,
..,..,.. - -, - 2008 378.146(075.8) -481.28 73 69 69.. - : /..,..,... : - -, 2008. 204. ISBN 5-98298-269-5. - -,, -.,,, -., -. - «- -»,. 378.146(075.8) -481.28 73 -,..,.. ISBN 5-98298-269-5..,..,.., 2008,
(a b) c = a (b c) e a e = e a = a. a a 1 = a 1 a = e. m+n
 Z 6 D 3 G = {a, b, c,... } G a, b G a b = c c (a b) c = a (b c) e a e = e a = a a a 1 = a 1 a = e Q = {0, ±1, ±2,..., ±n,... } m, n m+n m + 0 = m m + ( m) = 0 Z N = {a n }, n = 1, 2... N N Z N = {1, ω,
Z 6 D 3 G = {a, b, c,... } G a, b G a b = c c (a b) c = a (b c) e a e = e a = a a a 1 = a 1 a = e Q = {0, ±1, ±2,..., ±n,... } m, n m+n m + 0 = m m + ( m) = 0 Z N = {a n }, n = 1, 2... N N Z N = {1, ω,
!!" #7 $39 %" (07) ..,..,.. $ 39. ) :. :, «(», «%», «%», «%» «%». & ,. ). & :..,. '.. ( () #*. );..,..'. + (# ).
 1 00 3 !!" 344#7 $39 %" 6181001 63(07) & : ' ( () #* ); ' + (# ) $ 39 ) : : 00 %" 6181001 63(07)!!" 344#7 «(» «%» «%» «%» «%» & ) 4 )&-%/0 +- «)» * «1» «1» «)» ) «(» «%» «%» + ) 30 «%» «%» )1+ / + : +3
1 00 3 !!" 344#7 $39 %" 6181001 63(07) & : ' ( () #* ); ' + (# ) $ 39 ) : : 00 %" 6181001 63(07)!!" 344#7 «(» «%» «%» «%» «%» & ) 4 )&-%/0 +- «)» * «1» «1» «)» ) «(» «%» «%» + ) 30 «%» «%» )1+ / + : +3
TeSys contactors a.c. coils for 3-pole contactors LC1-D
 References a.c. coils for 3-pole contactors LC1-D Control circuit voltage Average resistance Inductance of Reference (1) Weight Uc at 0 C ± 10 % closed circuit For 3-pole " contactors LC1-D09...D38 and
References a.c. coils for 3-pole contactors LC1-D Control circuit voltage Average resistance Inductance of Reference (1) Weight Uc at 0 C ± 10 % closed circuit For 3-pole " contactors LC1-D09...D38 and
MÉTHODES ET EXERCICES
 J.-M. MONIER I G. HABERER I C. LARDON MATHS PCSI PTSI MÉTHODES ET EXERCICES 4 e édition Création graphique de la couverture : Hokus Pokus Créations Dunod, 2018 11 rue Paul Bert, 92240 Malakoff www.dunod.com
J.-M. MONIER I G. HABERER I C. LARDON MATHS PCSI PTSI MÉTHODES ET EXERCICES 4 e édition Création graphique de la couverture : Hokus Pokus Créations Dunod, 2018 11 rue Paul Bert, 92240 Malakoff www.dunod.com
! "# " #!$ &'( )'&* $ ##!$2 $ $$ 829 #-#-$&2 %( $8&2(9 #."/-0"$23#(&&#
 ! "# " #!$ %""! &'( )'&* $!"#$% &$'#( )*+#'(,#* /$##+(#0 &1$( #& 23 #(&&# +, -. % ($4 ($4 ##!$2 $567 56 $$ 829 #-#-$&2 %( $8&2(9 #."/-0"$23#(&&# 6 < 6 6 6 66 6< <
! "# " #!$ %""! &'( )'&* $!"#$% &$'#( )*+#'(,#* /$##+(#0 &1$( #& 23 #(&&# +, -. % ($4 ($4 ##!$2 $567 56 $$ 829 #-#-$&2 %( $8&2(9 #."/-0"$23#(&&# 6 < 6 6 6 66 6< <
Ηράκλειο Κρήτης, 22 Ιουνίου 2018 (Παρασκευή)
 Ηράκλειο Κρήτης, 22 Ιουνίου 2018 (Παρασκευή) Επίπεδα А1, А2, В1, В2 (όλες οι ενότητες) Τόπος διεξαγωγής: Πανεπιστήμιο Κρήτης, Πανεπιστημιούπολη Βουτών, ΤΜΗΜΑ ΕΠΙΣΤΗΜΗΣ ΥΠΟΛΟΓΙΣΤΩΝ, ΑΜΦΙΘΕΑΤΡΟ Β, 2ο όροφο
Ηράκλειο Κρήτης, 22 Ιουνίου 2018 (Παρασκευή) Επίπεδα А1, А2, В1, В2 (όλες οι ενότητες) Τόπος διεξαγωγής: Πανεπιστήμιο Κρήτης, Πανεπιστημιούπολη Βουτών, ΤΜΗΜΑ ΕΠΙΣΤΗΜΗΣ ΥΠΟΛΟΓΙΣΤΩΝ, ΑΜΦΙΘΕΑΤΡΟ Β, 2ο όροφο
(x y) = (X = x Y = y) = (Y = y) (x y) = f X,Y (x, y) x f X
 X, Y f X,Y x, y X x, Y y f X Y x y X x Y y X x, Y y Y y f X,Y x, y f Y y f X Y x y x y X Y f X,Y x, y f X Y x y f X,Y x, y f Y y x y X : Ω R Y : Ω E X < y Y Y y 0 X Y y x R x f X Y x y gy X Y gy gy : Ω
X, Y f X,Y x, y X x, Y y f X Y x y X x Y y X x, Y y Y y f X,Y x, y f Y y f X Y x y x y X Y f X,Y x, y f X Y x y f X,Y x, y f Y y x y X : Ω R Y : Ω E X < y Y Y y 0 X Y y x R x f X Y x y gy X Y gy gy : Ω
2013/2012. m' Z (C) : V= (E): (C) :3,24 m/s. (A) : T= (1-z).g. (D) :4,54 m/s
 ( ) 03/0 - o l P z o M l =.P S. ( ) m' Z l=m m=kg m =,5Kg g=0/kg : : : : Q. (A) : V= (B) : V= () : V= (D) : V= (): : V :Q. (A) :4m/s (B) :0,4 m/s () :5m/s (D) :0,5m/s (): : M T : Q.3 (A) : T=(-z).g (B)
( ) 03/0 - o l P z o M l =.P S. ( ) m' Z l=m m=kg m =,5Kg g=0/kg : : : : Q. (A) : V= (B) : V= () : V= (D) : V= (): : V :Q. (A) :4m/s (B) :0,4 m/s () :5m/s (D) :0,5m/s (): : M T : Q.3 (A) : T=(-z).g (B)
 K K 1 2 1 K M N M(2 N 1) K K K K K f f(x 1, x 2,..., x K ) = K f xk (x k ), x 1, x 2,..., x K K K K f Yk (y k x 1, x 2,..., x k ) k=1 M i, i = 1, 2 Xi n n Yi n Xn 1 Xn 2 ˆM i P (n) e = {( ˆM 1, ˆM2 )
K K 1 2 1 K M N M(2 N 1) K K K K K f f(x 1, x 2,..., x K ) = K f xk (x k ), x 1, x 2,..., x K K K K f Yk (y k x 1, x 2,..., x k ) k=1 M i, i = 1, 2 Xi n n Yi n Xn 1 Xn 2 ˆM i P (n) e = {( ˆM 1, ˆM2 )
cz+d d (ac + cd )z + bc + dd c z + d
 T (z) = az + b cz + d ; a, b, c, d C, ad bc 0 ( ) a b M T (z) = (z) az + b c d cz + d (T T )(z) = T (T (z) (T T )(z) = az+b a + cz+d b c az+b + = (aa + cb )z + a b + b d a z + b cz+d d (ac + cd )z + bc
T (z) = az + b cz + d ; a, b, c, d C, ad bc 0 ( ) a b M T (z) = (z) az + b c d cz + d (T T )(z) = T (T (z) (T T )(z) = az+b a + cz+d b c az+b + = (aa + cb )z + a b + b d a z + b cz+d d (ac + cd )z + bc
Θερμότητα - διαφάνειες , Σειρά 1
 Θερμότητα - διαφάνειες 007-8, Σειρά Βιβλιογραφία (ενδεικτική) H.D. Young, Πανεπιστημιακή Φυσική Τόμος Α, (5-, 5-, 5-3, 5-5, 5-6, 6-, 6-, 6-4, 7-, 7-, 7-3, 7-4, 7-5, 7-6, 7-7,7-8) Σημειώσεις καθ. Κου Δ.
Θερμότητα - διαφάνειες 007-8, Σειρά Βιβλιογραφία (ενδεικτική) H.D. Young, Πανεπιστημιακή Φυσική Τόμος Α, (5-, 5-, 5-3, 5-5, 5-6, 6-, 6-, 6-4, 7-, 7-, 7-3, 7-4, 7-5, 7-6, 7-7,7-8) Σημειώσεις καθ. Κου Δ.
! "# $ % $&'& () *+ (,-. / 0 1(,21(,*) (3 4 5 "$ 6, ::: ;"<$& = = 7 + > + 5 $?"# 46(A *( / A 6 ( 1,*1 B"',CD77E *+ *),*,*) F? $G'& 0/ (,.
 ! " #$%&'()' *('+$,&'-. /0 1$23(/%/4. 1$)('%%'($( )/,)$5)/6%6 7$85,-9$(- /0 :/986-$, ;2'$(2$ 1'$-/-$)('')5( /&5&-/ 5(< =(4'($$,'(4 1$%$2/996('25-'/(& ;/0->5,$ 1'$-/%'')$(($/3?$%9'&-/?$( 5(< @6%-'9$
! " #$%&'()' *('+$,&'-. /0 1$23(/%/4. 1$)('%%'($( )/,)$5)/6%6 7$85,-9$(- /0 :/986-$, ;2'$(2$ 1'$-/-$)('')5( /&5&-/ 5(< =(4'($$,'(4 1$%$2/996('25-'/(& ;/0->5,$ 1'$-/%'')$(($/3?$%9'&-/?$( 5(< @6%-'9$
! " #$% & '()()*+.,/0.
 ! " #$% & '()()*+,),--+.,/0. 1!!" "!! 21 # " $%!%!! &'($ ) "! % " % *! 3 %,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,0 %%4,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,5
! " #$% & '()()*+,),--+.,/0. 1!!" "!! 21 # " $%!%!! &'($ ) "! % " % *! 3 %,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,0 %%4,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,5
lim Δt Δt 0 da da da dt dt dt dt Αν ο χρόνος αυξηθεί κατά Δt το διάνυσμα θα γίνει Εξετάζουμε την παράσταση
 Έστω διάνυσμα a( t a ( t i a ( t j a ( t k Αν ο χρόνος αυξηθεί κατά Δt το διάνυσμα θα γίνει a( t Δt a ( t Δt i a ( t Δt j a ( t Δt k Εξετάζουμε την παράσταση z z a( t Δt - a( t Δa a ( t Δt - a ( t lim
Έστω διάνυσμα a( t a ( t i a ( t j a ( t k Αν ο χρόνος αυξηθεί κατά Δt το διάνυσμα θα γίνει a( t Δt a ( t Δt i a ( t Δt j a ( t Δt k Εξετάζουμε την παράσταση z z a( t Δt - a( t Δa a ( t Δt - a ( t lim
2. Α ν ά λ υ σ η Π ε ρ ι ο χ ή ς. 3. Α π α ι τ ή σ ε ι ς Ε ρ γ ο δ ό τ η. 4. Τ υ π ο λ ο γ ί α κ τ ι ρ ί ω ν. 5. Π ρ ό τ α σ η. 6.
 Π Ε Ρ Ι Ε Χ Ο Μ Ε Ν Α 1. Ε ι σ α γ ω γ ή 2. Α ν ά λ υ σ η Π ε ρ ι ο χ ή ς 3. Α π α ι τ ή σ ε ι ς Ε ρ γ ο δ ό τ η 4. Τ υ π ο λ ο γ ί α κ τ ι ρ ί ω ν 5. Π ρ ό τ α σ η 6. Τ ο γ ρ α φ ε ί ο 1. Ε ι σ α γ ω
Π Ε Ρ Ι Ε Χ Ο Μ Ε Ν Α 1. Ε ι σ α γ ω γ ή 2. Α ν ά λ υ σ η Π ε ρ ι ο χ ή ς 3. Α π α ι τ ή σ ε ι ς Ε ρ γ ο δ ό τ η 4. Τ υ π ο λ ο γ ί α κ τ ι ρ ί ω ν 5. Π ρ ό τ α σ η 6. Τ ο γ ρ α φ ε ί ο 1. Ε ι σ α γ ω
ΕΡΓΑΣΙΑ 6. Ημερομηνία Παράδοσης: 29/6/09
 ΕΡΓΑΣΙΑ 6 Ημερομηνία Παράδοσης: 9/6/9 1. Ένας ομογενώς φορτισμένος μονωτικός κυκλικός δίσκος ακτίνας με συνολικό φορτίο τοποθετείται στο επίπεδο xy. Να βρείτε το ηλεκτρικό πεδίο σε σημείο P που βρίσκεται
ΕΡΓΑΣΙΑ 6 Ημερομηνία Παράδοσης: 9/6/9 1. Ένας ομογενώς φορτισμένος μονωτικός κυκλικός δίσκος ακτίνας με συνολικό φορτίο τοποθετείται στο επίπεδο xy. Να βρείτε το ηλεκτρικό πεδίο σε σημείο P που βρίσκεται
 1951 {0, 1} N = N \ {0} n m M n, m N F x i = (x i 1,..., xi m) x j = (x 1 j,..., xn j ) i j M M i j x i j m n M M M M T f : F m F f(m) f M (f(x 1 1,..., x1 m),..., f(x n 1,..., xn m)) T R F M R M R x
1951 {0, 1} N = N \ {0} n m M n, m N F x i = (x i 1,..., xi m) x j = (x 1 j,..., xn j ) i j M M i j x i j m n M M M M T f : F m F f(m) f M (f(x 1 1,..., x1 m),..., f(x n 1,..., xn m)) T R F M R M R x
J! "#$ %"& ( ) ) ) " *+, -./0-, *- /! /!+12, ,. 6 /72-, 0,,3-8 / ',913-51:-*/;+ 5/<3/ +15;+ 5/<3=9 -!.1!-9 +17/> ) ) &
 J! "#$ %"& J ' ( ) ) ) " *+, -./0-, L *- /! /!+12,3-4 % +15,. 6 /72-, 0,,3-8 / ',913-51:-*/;+ 5/01 ',913-51:--
J! "#$ %"& J ' ( ) ) ) " *+, -./0-, L *- /! /!+12,3-4 % +15,. 6 /72-, 0,,3-8 / ',913-51:-*/;+ 5/01 ',913-51:--
 V r,k j F k m N k+1 N k N k+1 H j n = 7 n = 16 Ṽ r ñ,ñ j Ṽ Ṽ j x / Ṽ W 2r V r D N T T 2r 2r N k F k N 2r Ω R 2 n Ω I n = { N: n} n N R 2 x R 2, I n Ω R 2 u R 2, I n x k+1 = x k + u k, u, x R 2,
V r,k j F k m N k+1 N k N k+1 H j n = 7 n = 16 Ṽ r ñ,ñ j Ṽ Ṽ j x / Ṽ W 2r V r D N T T 2r 2r N k F k N 2r Ω R 2 n Ω I n = { N: n} n N R 2 x R 2, I n Ω R 2 u R 2, I n x k+1 = x k + u k, u, x R 2,
!! " &' ': " /.., c #$% & - & ' ()",..., * +,.. * ' + * - - * ()",...(.
 ..,.. 00 !!.6 7 " 57 +: #$% & - & ' ()",..., * +,.. * ' + * - - * ()",.....(. 8.. &' ': " /..,... :, 00. c. " *+ ' * ' * +' * - * «/'» ' - &, $%' * *& 300.65 «, + *'». 3000400- -00 3-00.6, 006 3 4.!"#"$
..,.. 00 !!.6 7 " 57 +: #$% & - & ' ()",..., * +,.. * ' + * - - * ()",.....(. 8.. &' ': " /..,... :, 00. c. " *+ ' * ' * +' * - * «/'» ' - &, $%' * *& 300.65 «, + *'». 3000400- -00 3-00.6, 006 3 4.!"#"$
m 1, m 2 F 12, F 21 F12 = F 21
 m 1, m 2 F 12, F 21 F12 = F 21 r 1, r 2 r = r 1 r 2 = r 1 r 2 ê r = rê r F 12 = f(r)ê r F 21 = f(r)ê r f(r) f(r) < 0 f(r) > 0 m 1 r1 = f(r)ê r m 2 r2 = f(r)ê r r = r 1 r 2 r 1 = 1 m 1 f(r)ê r r 2 = 1 m
m 1, m 2 F 12, F 21 F12 = F 21 r 1, r 2 r = r 1 r 2 = r 1 r 2 ê r = rê r F 12 = f(r)ê r F 21 = f(r)ê r f(r) f(r) < 0 f(r) > 0 m 1 r1 = f(r)ê r m 2 r2 = f(r)ê r r = r 1 r 2 r 1 = 1 m 1 f(r)ê r r 2 = 1 m
!"#$ "%&$ ##%&%'()) *..$ /. 0-1$ )$.'-
 !!" !"# "%& ##%&%',-... /. -1.'- -13-',,'- '-...4 %. -5"'-1.... /..'-1.....-"..'-1.. 78::8
!!" !"# "%& ##%&%',-... /. -1.'- -13-',,'- '-...4 %. -5"'-1.... /..'-1.....-"..'-1.. 78::8
Εισαγωγή στην Αστρόβιλη Άκυκλη Ροή
 ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΤΜΗΜΑ ΜΗΧΑΝΟΛΟΓΩΝ ΜΗΧΑΝΙΚΩΝ ΤΟΜΕΑΣ ΒΙΟΜΗΧΑΝΙΚΗΣ ΔΙΟΙΚΗΣΗΣ ΚΑΙ ΕΠΙΧΕΙΡΗΣΙΑΚΗΣ ΕΡΕΥΝΑΣ ΑΕΡΟΔΥΝΑΜΙΚΗ Διδάσκων: Δρ. Ριζιώτης Βασίλης Εισαγωγή στην Αστρόβιλη Άκυκλη Ροή Άδεια Χρήσης
ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΤΜΗΜΑ ΜΗΧΑΝΟΛΟΓΩΝ ΜΗΧΑΝΙΚΩΝ ΤΟΜΕΑΣ ΒΙΟΜΗΧΑΝΙΚΗΣ ΔΙΟΙΚΗΣΗΣ ΚΑΙ ΕΠΙΧΕΙΡΗΣΙΑΚΗΣ ΕΡΕΥΝΑΣ ΑΕΡΟΔΥΝΑΜΙΚΗ Διδάσκων: Δρ. Ριζιώτης Βασίλης Εισαγωγή στην Αστρόβιλη Άκυκλη Ροή Άδεια Χρήσης
!"#$%& '!(#)& a<.21c67.<9 /06 :6>/ 54.6: 1. ]1;A76 _F -. /06 4D26.36 <> A.:4D6:6C C4/4 /06 D:43? C</ O=47?6C b*dp 12 :1?6:E /< D6 3:4221N6C 42 D:A6 O=
![!#$%& '!(#)& a<.21c67.<9 /06 :6>/ 54.6: 1. ]1;A76 _F -. /06 4D26.36 <> A.:4D6:6C C4/4 /06 D:43? C</ O=47?6C b*dp 12 :1?6:E /< D6 3:4221N6C 42 D:A6 O= !#$%& '!(#)& a<.21c67.<9 /06 :6>/ 54.6: 1. ]1;A76 _F -. /06 4D26.36 <> A.:4D6:6C C4/4 /06 D:43? C</ O=47?6C b*dp 12 :1?6:E /< D6 3:4221N6C 42 D:A6 O=](/thumbs/72/67457957.jpg) ! " #$% & '( )*+, -. /012 3045/67 8 96 57626./ 4. 4:;74= 69676.36 D426C
! " #$% & '( )*+, -. /012 3045/67 8 96 57626./ 4. 4:;74= 69676.36 D426C
Κεφάλαιο 1 Πραγματικοί Αριθμοί 1.1 Σύνολα
 x + = 0 N = {,, 3....}, Z Q, b, b N c, d c, d N + b = c, b = d. N = =. < > P n P (n) P () n = P (n) P (n + ) n n + P (n) n P (n) n P n P (n) P (m) P (n) n m P (n + ) P (n) n m P n P (n) P () P (), P (),...,
x + = 0 N = {,, 3....}, Z Q, b, b N c, d c, d N + b = c, b = d. N = =. < > P n P (n) P () n = P (n) P (n + ) n n + P (n) n P (n) n P n P (n) P (m) P (n) n m P (n + ) P (n) n m P n P (n) P () P (), P (),...,
The one-dimensional periodic Schrödinger equation
 The one-dmensonal perodc Schrödnger equaon Jordan Bell jordan.bell@gmal.com Deparmen of Mahemacs, Unversy of Torono Aprl 23, 26 Translaons and convoluon For y, le τ y f(x f(x y. To say ha f : C s unformly
The one-dmensonal perodc Schrödnger equaon Jordan Bell jordan.bell@gmal.com Deparmen of Mahemacs, Unversy of Torono Aprl 23, 26 Translaons and convoluon For y, le τ y f(x f(x y. To say ha f : C s unformly
d 2 y dt 2 xdy dt + d2 x
 y t t ysin y d y + d y y t z + y ty yz yz t z y + t + y + y + t y + t + y + + 4 y 4 + t t + 5 t Ae cos + Be sin 5t + 7 5 y + t / m_nadjafikhah@iustacir http://webpagesiustacir/m_nadjafikhah/courses/ode/fa5pdf
y t t ysin y d y + d y y t z + y ty yz yz t z y + t + y + y + t y + t + y + + 4 y 4 + t t + 5 t Ae cos + Be sin 5t + 7 5 y + t / m_nadjafikhah@iustacir http://webpagesiustacir/m_nadjafikhah/courses/ode/fa5pdf
Points de torsion des courbes elliptiques et équations diophantiennes
 Points de torsion des courbes elliptiques et équations diophantiennes Nicolas Billerey To cite this version: Nicolas Billerey. Points de torsion des courbes elliptiques et équations diophantiennes. Mathématiques
Points de torsion des courbes elliptiques et équations diophantiennes Nicolas Billerey To cite this version: Nicolas Billerey. Points de torsion des courbes elliptiques et équations diophantiennes. Mathématiques
-! " #!$ %& ' %( #! )! ' 2003
 -! "#!$ %&' %(#!)!' ! 7 #!$# 9 " # 6 $!% 6!!! 6! 6! 6 7 7 &! % 7 ' (&$ 8 9! 9!- "!!- ) % -! " 6 %!( 6 6 / 6 6 7 6!! 7 6! # 8 6!! 66! #! $ - (( 6 6 $ % 7 7 $ 9!" $& & " $! / % " 6!$ 6!!$#/ 6 #!!$! 9 /!
-! "#!$ %&' %(#!)!' ! 7 #!$# 9 " # 6 $!% 6!!! 6! 6! 6 7 7 &! % 7 ' (&$ 8 9! 9!- "!!- ) % -! " 6 %!( 6 6 / 6 6 7 6!! 7 6! # 8 6!! 66! #! $ - (( 6 6 $ % 7 7 $ 9!" $& & " $! / % " 6!$ 6!!$#/ 6 #!!$! 9 /!
Erkki Mäkinen ja Timo Poranen Algoritmit
 rkki Mäkinen ja Timo Poranen Algoritmit TITOJNKÄSITTLYTITIDN LAITOS TAMPRN YLIOPISTO D 2008 6 TAMPR 2009 TAMPRN YLIOPISTO TITOJNKÄSITTLYTITIDN LAITOS JULKAISUSARJA D VRKKOJULKAISUT D 2008 6, TOUKOKUU 2009
rkki Mäkinen ja Timo Poranen Algoritmit TITOJNKÄSITTLYTITIDN LAITOS TAMPRN YLIOPISTO D 2008 6 TAMPR 2009 TAMPRN YLIOPISTO TITOJNKÄSITTLYTITIDN LAITOS JULKAISUSARJA D VRKKOJULKAISUT D 2008 6, TOUKOKUU 2009
 a; b 2 R; a < b; f : [a; b] R! R y 2 R: y : [a; b]! R; ( y (t) = f t; y(t) ; a t b; y(a) = y : f (t; y) 2 [a; b]r: f 2 C ([a; b]r): y 2 C [a; b]; y(a) = y ; f y ỹ ỹ y ; jy ỹ j ky ỹk [a; b]; f y; ( y (t)
a; b 2 R; a < b; f : [a; b] R! R y 2 R: y : [a; b]! R; ( y (t) = f t; y(t) ; a t b; y(a) = y : f (t; y) 2 [a; b]r: f 2 C ([a; b]r): y 2 C [a; b]; y(a) = y ; f y ỹ ỹ y ; jy ỹ j ky ỹk [a; b]; f y; ( y (t)
A 1 A 2 A 3 B 1 B 2 B 3
 16 0 17 0 17 0 18 0 18 0 19 0 20 A A = A 1 î + A 2 ĵ + A 3ˆk A (x, y, z) r = xî + yĵ + zˆk A B A B B A = A 1 B 1 + A 2 B 2 + A 3 B 3 = A B θ θ A B = ˆn A B θ A B î ĵ ˆk = A 1 A 2 A 3 B 1 B 2 B 3 W = F
16 0 17 0 17 0 18 0 18 0 19 0 20 A A = A 1 î + A 2 ĵ + A 3ˆk A (x, y, z) r = xî + yĵ + zˆk A B A B B A = A 1 B 1 + A 2 B 2 + A 3 B 3 = A B θ θ A B = ˆn A B θ A B î ĵ ˆk = A 1 A 2 A 3 B 1 B 2 B 3 W = F
 γ n ϑ n n ψ T 8 Q 6 j, k, m, n, p, r, r t, x, y f m (x) (f(x)) m / a/b (f g)(x) = f(g(x)) n f f n I J α β I = α + βj N, Z, Q ϕ Εὐκλείδης ὁ Ἀλεξανδρεύς Στοιχεῖα ἄκρος καὶ μέσος λόγος ὕδωρ αἰθήρ ϕ φ Φ τ
γ n ϑ n n ψ T 8 Q 6 j, k, m, n, p, r, r t, x, y f m (x) (f(x)) m / a/b (f g)(x) = f(g(x)) n f f n I J α β I = α + βj N, Z, Q ϕ Εὐκλείδης ὁ Ἀλεξανδρεύς Στοιχεῖα ἄκρος καὶ μέσος λόγος ὕδωρ αἰθήρ ϕ φ Φ τ
ΑΝΑΛΥΣΗ ΙΙ- ΠΟΛΙΤΙΚΟΙ ΜΗΧΑΝΙΚΟΙ ΦΥΛΛΑΔΙΟ 2/2012
 ΑΝΑΛΥΣΗ ΙΙ- ΠΟΛΙΤΙΚΟΙ ΜΗΧΑΝΙΚΟΙ ΦΥΛΛΑΔΙΟ /0 Έστω r rx, y, z, I a, b συνάρτηση C τάξης και r r r x y z Nα αποδείξετε ότι: d dr r (α) r r, I r r r d dr d r (β) r r, I dr (γ) Αν r 0, για κάθε I κάθε I d (δ)
ΑΝΑΛΥΣΗ ΙΙ- ΠΟΛΙΤΙΚΟΙ ΜΗΧΑΝΙΚΟΙ ΦΥΛΛΑΔΙΟ /0 Έστω r rx, y, z, I a, b συνάρτηση C τάξης και r r r x y z Nα αποδείξετε ότι: d dr r (α) r r, I r r r d dr d r (β) r r, I dr (γ) Αν r 0, για κάθε I κάθε I d (δ)
Προβολές και Μετασχηματισμοί Παρατήρησης
 Γραφικά & Οπτικοποίηση Κεφάλαιο 4 Προβολές και Μετασχηματισμοί Παρατήρησης Εισαγωγή Στα γραφικά υπάρχουν: 3Δ μοντέλα 2Δ συσκευές επισκόπησης (οθόνες & εκτυπωτές) Προοπτική απεικόνιση (προβολή): Λαμβάνει
Γραφικά & Οπτικοποίηση Κεφάλαιο 4 Προβολές και Μετασχηματισμοί Παρατήρησης Εισαγωγή Στα γραφικά υπάρχουν: 3Δ μοντέλα 2Δ συσκευές επισκόπησης (οθόνες & εκτυπωτές) Προοπτική απεικόνιση (προβολή): Λαμβάνει
!"#!$% &' ( )*+*,% $ &$ -.&01#(2$#3 4-$ #35667
 !"#!$% & &' ( )*+*,% $ -*(-$ -.*/% $- &$ -.&01#(2$#3 4-$ #35667 5051 & 00000000000000000000000000000000000000000000000000000000000000000000000000000 9 508&:;&& 0000000000000000000000000000000000000000000000000
!"#!$% & &' ( )*+*,% $ -*(-$ -.*/% $- &$ -.&01#(2$#3 4-$ #35667 5051 & 00000000000000000000000000000000000000000000000000000000000000000000000000000 9 508&:;&& 0000000000000000000000000000000000000000000000000
JMAK の式の一般化と粒子サイズ分布の計算 by T.Koyama
 MAK by T.Koyama MAK MAK f () = exp{ fex () = exp (') v(, ') ' () (') ' v (, ') ' f (), (), v (, ') f () () f () () v (, ') f () () v (, ') f () () () = + {exp( A) () f () = exp( K ) () K,,, A *** ***************************************************************************
MAK by T.Koyama MAK MAK f () = exp{ fex () = exp (') v(, ') ' () (') ' v (, ') ' f (), (), v (, ') f () () f () () v (, ') f () () v (, ') f () () () = + {exp( A) () f () = exp( K ) () K,,, A *** ***************************************************************************
Αυτό το κεφάλαιο εξηγεί τις ΠΑΡΑΜΕΤΡΟΥΣ προς χρήση αυτού του προϊόντος. Πάντα να μελετάτε αυτές τις οδηγίες πριν την χρήση.
 Αυτό το κεφάλαιο εξηγεί τις ΠΑΡΑΜΕΤΡΟΥΣ προς χρήση αυτού του προϊόντος. Πάντα να μελετάτε αυτές τις οδηγίες πριν την χρήση. 3. Λίστα Παραμέτρων 3.. Λίστα Παραμέτρων Στην αρχική ρύθμιση, μόνο οι παράμετροι
Αυτό το κεφάλαιο εξηγεί τις ΠΑΡΑΜΕΤΡΟΥΣ προς χρήση αυτού του προϊόντος. Πάντα να μελετάτε αυτές τις οδηγίες πριν την χρήση. 3. Λίστα Παραμέτρων 3.. Λίστα Παραμέτρων Στην αρχική ρύθμιση, μόνο οι παράμετροι
Inflation and Reheating in Spontaneously Generated Gravity
 Univesità di Bologna Inflation and Reheating in Spontaneously Geneated Gavity (A. Ceioni, F. Finelli, A. Tonconi, G. Ventui) Phys.Rev.D81:123505,2010 Motivations Inflation (FTV Phys.Lett.B681:383-386,2009)
Univesità di Bologna Inflation and Reheating in Spontaneously Geneated Gavity (A. Ceioni, F. Finelli, A. Tonconi, G. Ventui) Phys.Rev.D81:123505,2010 Motivations Inflation (FTV Phys.Lett.B681:383-386,2009)
ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΤΟΜΕΑΣ ΟΜΟΣΤΑΤΙΚΗΣ & ΑΝΤΙΣΕΙΣΜΙΚΩΝ ΕΡΕΥΝΩΝ ΘΕΩΡΙΑ ΚΕΛΥΦΩΝ. Καθ. Βλάσης Κουµούσης
 ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΤΟΜΕΑΣ ΟΜΟΣΤΑΤΙΚΗΣ & ΑΝΤΙΣΕΙΣΜΙΚΩΝ ΕΡΕΥΝΩΝ ΘΕΩΡΙΑ ΚΕΛΥΦΩΝ Καθ. Βλάσης Κουµούσης Θεµελιώδες Θεώρηµα Θεωρίας Επιφανειών Αφορά στην ανάπτυξη τριών διαφορετικών εξισώσεων (Gauss-Cdazzi)
ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΤΟΜΕΑΣ ΟΜΟΣΤΑΤΙΚΗΣ & ΑΝΤΙΣΕΙΣΜΙΚΩΝ ΕΡΕΥΝΩΝ ΘΕΩΡΙΑ ΚΕΛΥΦΩΝ Καθ. Βλάσης Κουµούσης Θεµελιώδες Θεώρηµα Θεωρίας Επιφανειών Αφορά στην ανάπτυξη τριών διαφορετικών εξισώσεων (Gauss-Cdazzi)
,, #,#, %&'(($#(#)&*"& 3,,#!4!4! +&'(#,-$#,./$012 5 # # %, )
 !! "#$%&'%( (%)###**#+!"#$ ',##-.#,,, #,#, /01('/01/'#!2#! %&'(($#(#)&*"& 3,,#!4!4! +&'(#,-$#,./$012 5 # # %, ) 6###+! 4! 4! 4,*!47! 4! (! 8!9%,,#!41! 4! (! 4!5),!(8! 4! (! :!;!(7! (! 4! 4!!8! (! 8! 4!!8(!44!
!! "#$%&'%( (%)###**#+!"#$ ',##-.#,,, #,#, /01('/01/'#!2#! %&'(($#(#)&*"& 3,,#!4!4! +&'(#,-$#,./$012 5 # # %, ) 6###+! 4! 4! 4,*!47! 4! (! 8!9%,,#!41! 4! (! 4!5),!(8! 4! (! :!;!(7! (! 4! 4!!8! (! 8! 4!!8(!44!
Ax = b. 7x = 21. x = 21 7 = 3.
 3 s st 3 r 3 t r 3 3 t s st t 3t s 3 3 r 3 3 st t t r 3 s t t r r r t st t rr 3t r t 3 3 rt3 3 t 3 3 r st 3 t 3 tr 3 r t3 t 3 s st t Ax = b. s t 3 t 3 3 r r t n r A tr 3 rr t 3 t n ts b 3 t t r r t x 3
3 s st 3 r 3 t r 3 3 t s st t 3t s 3 3 r 3 3 st t t r 3 s t t r r r t st t rr 3t r t 3 3 rt3 3 t 3 3 r st 3 t 3 tr 3 r t3 t 3 s st t Ax = b. s t 3 t 3 3 r r t n r A tr 3 rr t 3 t n ts b 3 t t r r t x 3
= +. 2 c = JK = evk, S E V V ( ) 1 2
 Σ α Μηχα, Ε ε α Ι υ υ, 6/6/. ( α ο Η υ α υ α υ υ Ising π β α απ φ α ( β Q e e = +. (α α φ α π α ( S / α α ( C/ α spin υ α α α α πα α υ. ( α απ π α αφ α α α α α S / α C/ α α >.. ( α ο.5 Α α π υ α Landau
Σ α Μηχα, Ε ε α Ι υ υ, 6/6/. ( α ο Η υ α υ α υ υ Ising π β α απ φ α ( β Q e e = +. (α α φ α π α ( S / α α ( C/ α spin υ α α α α πα α υ. ( α απ π α αφ α α α α α S / α C/ α α >.. ( α ο.5 Α α π υ α Landau
Ενότητα 4: Εισαγωγή στο Λογισμό Μεταβολών. Νίκος Καραμπετάκης Τμήμα Μαθηματικών
 ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΑΝΟΙΧΤΑ ΑΚΑΔΗΜΑΙΚΑ ΜΑΘΗΜΑΤΑ Ενότητα 4: Εισαγωγή στο Λογισμό Μεταβολών Νίκος Καραμπετάκης Το παρόν εκπαιδευτικό υλικό υπόκειται σε άδειες χρήσης Creative Commons.
ΑΡΙΣΤΟΤΕΛΕΙΟ ΠΑΝΕΠΙΣΤΗΜΙΟ ΘΕΣΣΑΛΟΝΙΚΗΣ ΑΝΟΙΧΤΑ ΑΚΑΔΗΜΑΙΚΑ ΜΑΘΗΜΑΤΑ Ενότητα 4: Εισαγωγή στο Λογισμό Μεταβολών Νίκος Καραμπετάκης Το παρόν εκπαιδευτικό υλικό υπόκειται σε άδειες χρήσης Creative Commons.
 a; b 2 R; a < b; f : [a; b] R! R y 2 R: y : [a; b]! R; ( y (t) = f t; y(t) ; a t b; y(a) = y : f (t; y) 2 [a; b]r: f 2 C ([a; b]r): y 2 C [a; b]; y(a) = y ; f y ỹ ỹ y ; jy ỹ j ky ỹk [a; b]; f y; ( y (t)
a; b 2 R; a < b; f : [a; b] R! R y 2 R: y : [a; b]! R; ( y (t) = f t; y(t) ; a t b; y(a) = y : f (t; y) 2 [a; b]r: f 2 C ([a; b]r): y 2 C [a; b]; y(a) = y ; f y ỹ ỹ y ; jy ỹ j ky ỹk [a; b]; f y; ( y (t)
/&25*+* 24.&6,2(2**02)' 24
 !! "#$ % (33 &' ())**,"-.&/(,01.2(*(33*( ( &,.*(33*( ( 2&/((,*(33*( 24 /&25** 24.&6,2(2**02)' 24 " 0 " ( 78,' 4 (33 72"08 " 2/((,02..2(& (902)' 4 #% 7' 2"8(7 39$:80(& 2/((,* (33; (* 3: &
!! "#$ % (33 &' ())**,"-.&/(,01.2(*(33*( ( &,.*(33*( ( 2&/((,*(33*( 24 /&25** 24.&6,2(2**02)' 24 " 0 " ( 78,' 4 (33 72"08 " 2/((,02..2(& (902)' 4 #% 7' 2"8(7 39$:80(& 2/((,* (33; (* 3: &
u = 0 u = ϕ t + Π) = 0 t + Π = C(t) C(t) C(t) = K K C(t) ϕ = ϕ 1 + C(t) dt Kt 2 ϕ = 0
 u = (u, v, w) ω ω = u = 0 ϕ u u = ϕ u = 0 ϕ 2 ϕ = 0 u t = u ω 1 ρ Π + ν 2 u Π = p + (1/2)ρ u 2 + ρgz ω = 0 ( ϕ t + Π) = 0 ϕ t + Π = C(t) C(t) C(t) = K K C(t) ϕ = ϕ 1 + C(t) dt Kt C(t) ϕ ϕ 1 ϕ = ϕ 1 p ρ
u = (u, v, w) ω ω = u = 0 ϕ u u = ϕ u = 0 ϕ 2 ϕ = 0 u t = u ω 1 ρ Π + ν 2 u Π = p + (1/2)ρ u 2 + ρgz ω = 0 ( ϕ t + Π) = 0 ϕ t + Π = C(t) C(t) C(t) = K K C(t) ϕ = ϕ 1 + C(t) dt Kt C(t) ϕ ϕ 1 ϕ = ϕ 1 p ρ
Free Energy Calculation
 Free Energy Calculation 1 Outline Free energies: classical thermodynamics Free energies: statistical thermodynamics Monte Carlo simulations: what went wrong? Experiments: how to make a chemical potentialmeter?
Free Energy Calculation 1 Outline Free energies: classical thermodynamics Free energies: statistical thermodynamics Monte Carlo simulations: what went wrong? Experiments: how to make a chemical potentialmeter?
f(w) f(z) = C f(z) = z z + h z h = h h h 0,h C f(z + h) f(z)
 Ω f: Ω C l C z Ω f f(w) f(z) z a w z = h 0,h C f(z + h) f(z) h = l. z f l = f (z) Ω f Ω f Ω H(Ω) n N C f(z) = z n h h 0 h z + h z h = h h C f(z) = z f (z) = f( z) f f: Ω C Ω = { z; z Ω} z, a Ω f (z) f
Ω f: Ω C l C z Ω f f(w) f(z) z a w z = h 0,h C f(z + h) f(z) h = l. z f l = f (z) Ω f Ω f Ω H(Ω) n N C f(z) = z n h h 0 h z + h z h = h h C f(z) = z f (z) = f( z) f f: Ω C Ω = { z; z Ω} z, a Ω f (z) f
ΑΤΜΟΣΦΑΙΡΙΚΗ ΘΕΡΜΟΔΥΝΑΜΙΚΗ. Η ατμόσφαιρα συμπεριφέρεται σαν ιδανικό αέριο (ειδικά για z>10 km)
 ΑΤΜΟΣΦΑΙΡΙΚΗ ΘΕΡΜΟΔΥΝΑΜΙΚΗ Η ατμόσφαιρα συμπεριφέρεται σαν ιδανικό αέριο (ειδικά για z>1 km) Οι αποστάσεις μεταξύ των μορίων είναι πολύ μεγάλες σχετικά με τον όγκο που κατέχουν Οι συγκρούσεις μεταξύ τους
ΑΤΜΟΣΦΑΙΡΙΚΗ ΘΕΡΜΟΔΥΝΑΜΙΚΗ Η ατμόσφαιρα συμπεριφέρεται σαν ιδανικό αέριο (ειδικά για z>1 km) Οι αποστάσεις μεταξύ των μορίων είναι πολύ μεγάλες σχετικά με τον όγκο που κατέχουν Οι συγκρούσεις μεταξύ τους
 Li % % % % % % % % % % 3d 4s V V V V d V V V n O V V V O V n O V n O % % X X % % % 10 10 cm Li Li Li LiMO 2 Li 1 x MO 2 + xl + 1 + xe C + xl + 1 + xe Li x C LiMO 2 +C Li x C + Li 1 x MO 2
Li % % % % % % % % % % 3d 4s V V V V d V V V n O V V V O V n O V n O % % X X % % % 10 10 cm Li Li Li LiMO 2 Li 1 x MO 2 + xl + 1 + xe C + xl + 1 + xe Li x C LiMO 2 +C Li x C + Li 1 x MO 2
Poroelastic modelling of the coupled mechanical moisture behaviour of wood
 Ma terias Sci ence & Technoog y Poroeastic modeing of the couped mechanica moisture behaviour of wood M. Dresser, D. Derome, R. Guyer and J. Carmeiet poroeastic modeing of wood - COST meeting October 00.
Ma terias Sci ence & Technoog y Poroeastic modeing of the couped mechanica moisture behaviour of wood M. Dresser, D. Derome, R. Guyer and J. Carmeiet poroeastic modeing of wood - COST meeting October 00.
Η κατανομή ορμής Από την στατιστική μηχανική, ο αριθμός των μικροσκοπικών καταστάσεων dn στο στοιχείο όγκου του χώρου των φάσεων d 3 p d 3 r είναι
 ΤομοντέλοτουαερίουFermi ΤομοντέλοαυτόδιατυπώθηκεαπότονHansBethe.ΥποθέτουμεότιZπρωτόνια και N νετρόνια(φερμιόνια) καταλαμβάνουν ανεξάρτητα τον πυρηνικό όγκο Ω. Οιαλληλεπιδράσειςμεταξύτωνσωματίων(πυρηνικήκαιCoulomb)αγνοούνται.
ΤομοντέλοτουαερίουFermi ΤομοντέλοαυτόδιατυπώθηκεαπότονHansBethe.ΥποθέτουμεότιZπρωτόνια και N νετρόνια(φερμιόνια) καταλαμβάνουν ανεξάρτητα τον πυρηνικό όγκο Ω. Οιαλληλεπιδράσειςμεταξύτωνσωματίων(πυρηνικήκαιCoulomb)αγνοούνται.
Καθ. Βλάσης Κουµούσης
 ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΤΟΜΕΑΣ ΟΜΟΣΤΑΤΙΚΗΣ & ΑΝΤΙΣΕΙΣΜΙΚΩΝ ΕΡΕΥΝΩΝ ΘΕΩΡΙΑ ΚΕΛΥΦΩΝ Καθ. Βλάσης Κουµούσης Κελύη Εκ Περιστροής Μεµβρανική Θεωρία Τυχαία Φόρτιση Ανάπτυξη όρτισης σε σειρές Fourier: ( )
ΕΘΝΙΚΟ ΜΕΤΣΟΒΙΟ ΠΟΛΥΤΕΧΝΕΙΟ ΤΟΜΕΑΣ ΟΜΟΣΤΑΤΙΚΗΣ & ΑΝΤΙΣΕΙΣΜΙΚΩΝ ΕΡΕΥΝΩΝ ΘΕΩΡΙΑ ΚΕΛΥΦΩΝ Καθ. Βλάσης Κουµούσης Κελύη Εκ Περιστροής Μεµβρανική Θεωρία Τυχαία Φόρτιση Ανάπτυξη όρτισης σε σειρές Fourier: ( )
HONDA. Έτος κατασκευής
 Accord + Coupe IV 2.0 16V (CB3) F20A2-A3 81 110 01/90-09/93 0800-0175 11,00 2.0 16V (CB3) F20A6 66 90 01/90-09/93 0800-0175 11,00 2.0i 16V (CB3-CC9) F20A8 98 133 01/90-09/93 0802-9205M 237,40 2.0i 16V
Accord + Coupe IV 2.0 16V (CB3) F20A2-A3 81 110 01/90-09/93 0800-0175 11,00 2.0 16V (CB3) F20A6 66 90 01/90-09/93 0800-0175 11,00 2.0i 16V (CB3-CC9) F20A8 98 133 01/90-09/93 0802-9205M 237,40 2.0i 16V
την κεντρώα έκφραση πεπερασμένων διαφορών 2 ης τάξης και για τη παράγωγο f την ανάδρομη έκφραση πεπερασμένων διαφορών 2 ης τάξης xxx
 ΑΡΙΘΜΗΤΙΚΕΣ ΜΕΘΟΔΟΙ, 0-0, Ο ΕΞΑΜΗΝΟ ΕΡΓΑΣΙΑ #: ΑΡΙΘΜΗΤΙΚΗ ΠΑΡΑΓΩΓΙΣΗ και ΟΛΟΚΛΗΡΩΣΗ Ημερομηνία παράδοσης --0 Επιμέλεια απαντήσεων: Ιωάννης Λυχναρόπουλος ΑΣΚΗΣΗ Με βάση τη σειρά Taylor βρείτε για τη παράγωγο
ΑΡΙΘΜΗΤΙΚΕΣ ΜΕΘΟΔΟΙ, 0-0, Ο ΕΞΑΜΗΝΟ ΕΡΓΑΣΙΑ #: ΑΡΙΘΜΗΤΙΚΗ ΠΑΡΑΓΩΓΙΣΗ και ΟΛΟΚΛΗΡΩΣΗ Ημερομηνία παράδοσης --0 Επιμέλεια απαντήσεων: Ιωάννης Λυχναρόπουλος ΑΣΚΗΣΗ Με βάση τη σειρά Taylor βρείτε για τη παράγωγο
